Are you struggling with frequent titanium anode replacements and unexpected downtime in your industrial processes? This can be a real headache, leading to increased costs and decreased efficiency.
Titanium anodes are known for their corrosion resistance, but the following five factors affect its service life: current density, solution environment, temperature, Coating thickness, and regular cleaning. Understanding and managing these factors is key to maximizing their service life.

But don’t worry, I’m here to walk you through some practical solutions. We’ll delve into the top five factors that influence titanium anode corrosion, offering actionable advice to help you extend their lifespan and optimize your operations.
How to actively monitor titanium anode corrosion in industrial applications?
Worried about not catching corrosion signs early enough, leading to sudden shutdowns and costly repairs? It’s a valid concern; nobody wants unexpected downtime.
The best approach is real-time monitoring using electrochemical impedance spectroscopy (EIS) and potential measurements, coupled with periodic non-destructive testing (NDT) like XRD for coating analysis.1

Diving Deeper into Active Monitoring
Let’s break down why these methods are so effective and how you can implement them:
-
Electrochemical Impedance Spectroscopy (EIS)2:
- Think of EIS as an "EKG" for your anode. It sends small AC signals through the anode and measures the response.
- Changes in the impedance signal indicate changes in the anode’s surface, like coating degradation or the formation of a passive layer.
- By tracking these changes over time, you can predict when maintenance or replacement is needed before a failure occurs.
-
- This is a simpler, yet still valuable, technique. It involves continuously measuring the anode’s potential (voltage) relative to a reference electrode.
- Sudden shifts in potential can indicate corrosion activity or changes in the electrolyte.
-
Non-Destructive Testing (NDT) – XRD4:
- XRD (X-ray Diffraction) is like taking an X-ray of the anode’s coating.
- It reveals the crystal structure of the coating, allowing you to identify any changes or degradation that might be invisible to the naked eye.
- It is a perfect method for quality inspection.
| Monitoring Method | What it Measures | Benefits | Limitations |
|---|---|---|---|
| EIS | Impedance (resistance to AC current) | Highly sensitive to surface changes; can detect early signs of corrosion; quantitative data. | Requires specialized equipment and expertise; can be complex to interpret. |
| Potential Monitoring | Voltage relative to a reference electrode | Simple and inexpensive; continuous monitoring; easy to implement. | Less sensitive than EIS; provides less detailed information. |
| NDT (e.g., XRD, Ultrasound) | Coating structure, thickness, and defects | Non-destructive; can identify hidden problems; provides detailed information. | May require specialized equipment and expertise; typically done periodically, not continuously. |
Can coating thickness of iridium/ruthenium oxide directly impact corrosion resistance?
Are you trying to figure out the sweet spot for coating thickness2 – enough to protect, but not so much that you’re wasting money? Finding the right balance is crucial.
Yes, coating thickness5 significantly affects corrosion resistance. Industry standards typically recommend 2-5μm for IrO₂ coatings, but the optimal thickness depends on the specific operating conditions (acidic/alkaline environment).
Deeper Dive into Coating Thickness
Let’s get into the nitty-gritty of why thickness matters and how to choose the right one:
-
The "Critical Thickness":
- There’s a "critical thickness" for MMO (Mixed Metal Oxide1) coatings. Below this thickness, the coating may not provide adequate protection, leading to premature failure.
- Above this thickness, you may not see a significant increase in corrosion resistance, but you’ll be using (and paying for) more coating material.
-
Current Efficiency:
- Thicker coatings can sometimes decrease current efficiency. This means you’ll need to apply more current to achieve the desired electrochemical reaction, increasing energy consumption.
-
Matching Thickness to Conditions:
- Acidic environments often require that the coating should be thicker.
- Alkaline environments often require that the coating should be thinner.
| Environment | Coating Thickness Recommendation | Considerations |
|---|---|---|
| Acidic | 2-5μm (or thicker in harsh conditions) | Higher risk of coating dissolution; thicker coating provides more protection. |
| Alkaline | 2-5μm | Lower risk of coating dissolution (compared to acidic); balance thickness with current efficiency. |
| Neutral | 2-5μm | Consider other factors like electrolyte composition and temperature. |
| Highly Corrosive | >5μm | May require specialized coatings or multi-layer coatings for extreme conditions. The cost should be seriously considered. |
Does electrolyte contamination accelerate titanium anode failure? (e.g., chloride ions, organic matter)
Dealing with impurities in your electrolyte, and worried about how they might be speeding up anode failure? It is a common problem, especially in wastewater treatment.
Yes, electrolyte contamination6 significantly accelerates titanium anode failure. Chloride ions (Cl⁻) are particularly aggressive, but organic matter, sulfides, and other impurities can also contribute to corrosion.
Diving Deeper into Electrolyte Contamination
Let’s explore the mechanisms and solutions for different types of contaminants:
-
- Chloride ions are notorious for causing localized corrosion, like pitting and crevice corrosion.
- They can penetrate the passive layer on the titanium anode, leading to accelerated metal dissolution.
-
- Organic matter can adsorb onto the anode surface, blocking active sites and reducing efficiency.
- Some organic compounds can also be corrosive themselves.
-
Sulfides
- Sulfides are highly corrosive, they can cause rapid localized attack.
-
Filtration and Pretreatment:
- Effective filtration and pretreatment are essential for removing contaminants before they reach the anode.
- This might involve using filters, settling tanks, or chemical treatments.
-
Gradient Coatings:
- Gradient coatings, like Ti/RuO₂-IrO₂-Ta₂O₅, can enhance resistance to impurity corrosion.
- These coatings have a varying composition, with different layers optimized for different types of protection.
| Contaminant | Corrosion Mechanism | Filtration/Pretreatment Solutions |
|---|---|---|
| Chloride Ions (Cl⁻) | Pitting corrosion, crevice corrosion, breakdown of passive layer. | Reverse osmosis, ion exchange, specialized membranes. |
| Organic Matter | Adsorption, blocking of active sites, direct corrosion. | Activated carbon filtration, coagulation/flocculation, biological treatment. |
| Sulfides | Localized corrosion, accelerated metal dissolution | Chemical oxidation, precipitation, air stripping. |
| Heavy Metals | Deposition on anode surface, interference with electrochemical reactions. | Chemical precipitation, ion exchange, adsorption. |
How do temperature fluctuations affect the passivation layer stability of titanium anodes?
Are you experiencing significant temperature swings in your process, like during equipment startup and shutdown? This is a common challenge for many users.
Temperature fluctuations can significantly impact the stability of the passive layer on titanium anodes, potentially leading to cracking or spalling of the coating due to differences in thermal expansion.
Diving Deeper into Temperature Effects
Let’s explore the science behind this and how to mitigate the risks:
-
Coefficient of Thermal Expansion (CTE)9:
- The CTE describes how much a material expands or contracts with changes in temperature.
- Titanium (the substrate) and the oxide coating have different CTEs.
- When the temperature changes, these materials expand or contract at different rates, creating stress at the interface.
-
- Rapid temperature changes (thermal shock) can exacerbate this stress, leading to cracking or delamination of the coating.
-
Matching CTEs:
- Anode manufacturers strive to minimize the CTE mismatch between the substrate and the coating.
- This can be achieved through careful selection of coating materials and deposition techniques.
-
Thermal Shock10 Test
- Check the anode supplier with a thermal shock test report.
| Factor | Impact on Anode Stability | Mitigation Strategies |
|---|---|---|
| CTE Mismatch | Stress at the interface between substrate and coating, leading to cracking or delamination. | Choose anode materials with closely matched CTEs; use intermediate layers to buffer the stress. |
| Temperature Fluctuations | Cyclic stress, fatigue, and eventual failure of the coating. | Minimize temperature fluctuations11; use gradual heating and cooling protocols; choose anodes designed for thermal cycling resistance. |
| High Operating Temperatures | Accelerated corrosion rates, increased risk of coating degradation. | Operate within recommended temperature limits; consider using more thermally stable coating materials. |
Are sacrificial anode systems compatible with titanium anodes for enhanced protection?
Are you thinking about combining titanium anodes with sacrificial anodes for an extra layer of protection? Many people consider this approach, but it’s crucial to understand the compatibility.
Yes, sacrificial anode systems can be compatible with titanium anodes, but with caveats. Titanium anodes act as "non-consumable" anodes, while magnesium or zinc anodes sacrifice themselves. Careful design is needed to avoid potential reversal.
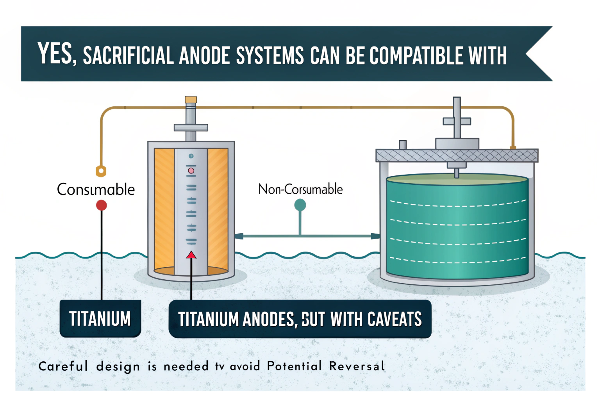
Diving Deeper into Hybrid Systems
Let’s unpack the complexities of combining these two types of protection:
-
How Sacrificial Anodes Work:
- Sacrificial anodes (typically zinc, magnesium, or aluminum) are more electrochemically active than titanium.
- They corrode preferentially, providing cathodic protection to the titanium anode and other metal structures.
-
Titanium Anodes as Non-Consumable:
- Titanium anodes, with their MMO coatings, are designed to be relatively inert.
- They facilitate the desired electrochemical reaction without being consumed themselves.
-
Hybrid Configurations:
- Hybrid systems are often used in complex environments, like submarine pipelines or offshore structures.
- It is important to electrically isolate.
-
Potential Reversal Risks:
- If the system is not designed correctly, there’s a risk of potential reversal.
- This can happen if the sacrificial anode becomes passivated or depleted, causing the titanium anode to become cathodic and corrode.
-
Intelligent Potential Controllers:
- To prevent potential reversal, intelligent potential controllers can be used.
- These controllers monitor the potential of the system and adjust the current output to maintain optimal protection.
| Consideration | Explanation | Solution/Recommendation |
|---|---|---|
| Compatibility | Titanium anodes and sacrificial anodes can work together, but careful design is needed. | Ensure proper electrical isolation and potential control. |
| Potential Reversal | Risk of titanium anode becoming cathodic and corroding if sacrificial anode fails. | Use intelligent potential controllers to monitor and adjust the system. |
| Application | Hybrid systems are suitable for complex environments where a single protection method is insufficient. | Conduct a thorough assessment of the environment and corrosion risks to determine if a hybrid system is necessary. |
| Maintenance | Sacrificial anodes require periodic replacement; titanium anodes have a longer lifespan but still need monitoring. | Develop a maintenance plan that includes regular inspection and replacement of sacrificial anodes. |
| Cost | Hybrid systems can be more expensive than using either method alone. | Consider the long-term costs and benefits, including the potential for reduced maintenance and extended lifespan. |
Conclusion
Extending the life of your titanium anodes requires a proactive approach, focusing on monitoring, coating optimization, electrolyte purity, temperature management, and, if necessary, a well-designed hybrid protection system. I’ve found that taking these steps can make a significant difference!
-
Learning about Mixed Metal Oxide coatings can provide insights into advanced corrosion protection technologies and their applications. ↩ ↩
-
Explore this link to understand how EIS can enhance your corrosion monitoring strategy and prevent costly failures. ↩ ↩
-
Discover how potential monitoring can provide continuous insights into corrosion activity, helping you maintain your systems effectively. ↩
-
Learn about the advantages of NDT and XRD in detecting hidden corrosion issues without damaging the anode. ↩
-
Understanding the impact of coating thickness on corrosion resistance can help optimize performance and cost-effectiveness. ↩
-
Understanding electrolyte contamination is crucial for improving anode longevity and efficiency in wastewater treatment. ↩
-
Chloride ions are a major factor in anode failure; exploring this can help mitigate corrosion risks. ↩
-
Organic matter can significantly impact anode performance; learning more can enhance treatment processes. ↩
-
Learn about the Coefficient of Thermal Expansion (CTE) for titanium to understand its behavior under temperature changes, which is crucial for material selection. ↩
-
Explore the concept of thermal shock and its effects on materials to better understand how to mitigate risks in your applications. ↩ ↩
-
Understanding the impact of temperature fluctuations on titanium anodes can help improve their performance and longevity. Explore this link for detailed insights. ↩





