Struggling with the persistent problem of metal corrosion? Feeling overwhelmed by the costs and dangers it brings? You’re not alone, this is a common issue.
Cathodic protection (CP) is a technique used to control the corrosion of a metal surface by making it the cathode of an electrochemical cell. This can be achieved by applying an external current or by connecting a sacrificial anode.

But, before we move on, Let’s break down everything in simple terms. Keep reading to get a clearer picture!
What is Cathodic Protection in Simple Terms?
Are you facing the headache of dealing with metal structures1 that constantly rust and corrode? Feeling frustrated by the ongoing maintenance?
In simple terms, cathodic protection2 is like giving your metal a bodyguard. It’s a method that uses electrical current to stop corrosion3, ensuring metal structures, like pipelines, stay strong and intact.
Understanding Corrosion
To get a better handle on CP, it is helpfull to first know how corrosion works. It is a complex electrochemical process. Here are key points:
- Electrochemical Nature: Metal corrosion happens when metal atoms lose electrons (oxidation) and form ions.
- Anodes and Cathodes: Metal surfaces have tiny areas acting like anodes (where corrosion occurs) and cathodes (where reduction occurs).
- Electrolyte: An electrolyte (like soil or water) is needed for ions to move and complete the circuit.
- Potential Difference: A difference in electrical potential between the anode and cathode drives the corrosion.
What is the Purpose of Cathodic Protection in a Pipeline?
Worried about your pipelines corroding and failing? The dangers of leaks and the cost of repairs can be a huge concern.
The main purpose of cathodic protection in a pipeline is to prevent corrosion. By applying a protective current, it keeps the pipeline from degrading, ensuring safety and extending its lifespan.
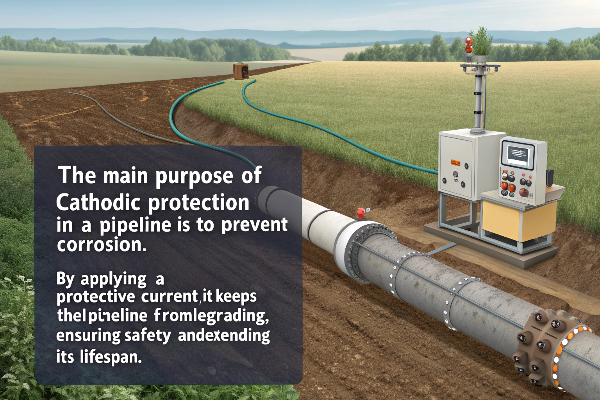
How Cathodic Protection Works on Pipelines
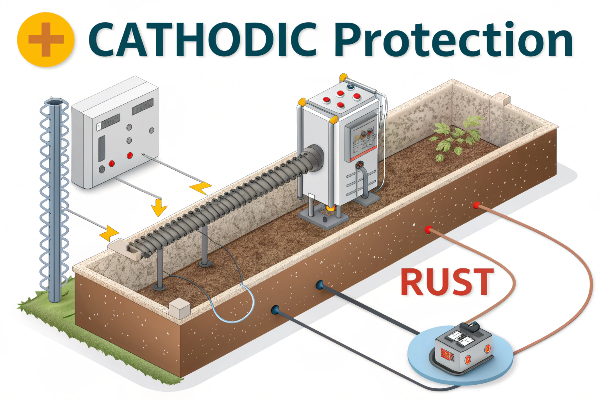
CP is like putting up an electrical shield around the metal. Let’s break down the key elements:
- Turning the Pipeline into a Cathode: The entire pipeline is forced to become the cathode of an electrochemical cell. This is the core idea.
- Two Main Methods:
- Sacrificial Anodes: More "active" metals (like zinc or magnesium) are connected to the pipeline. These corrode instead of the pipe.
- Impressed Current: An external power source (a rectifier) supplies a continuous protective current.
| Method | Description | Advantages | Disadvantages |
|---|---|---|---|
| Sacrificial Anode | Uses more reactive metals (Zn, Mg) that corrode preferentially. | Simple, no external power needed. | Limited driving potential, anodes need replacing. |
| Impressed Current | Uses an external DC power source to apply a protective current. | Can protect large structures, adjustable current output. | Requires power source, more complex, risk of stray current interference. |
How Does Cathodic Protection Prevent Rusting?
Concerned about rust4 eating away at your valuable metal structures? This can lead to weakening, failures, and costly repairs.
Cathodic protection prevents rusting by stopping the electrochemical process5 that causes it. It forces the metal to be the cathode, preventing the loss of metal ions, which is what forms rust.
Diving Deeper into the Science
Rust is the common term for iron oxide, formed when iron reacts with oxygen and water. It’s an oxidation process. Here is a deep look:
- Electron Control: CP works by controlling the flow of electrons. By supplying electrons to the metal, it suppresses the metal’s tendency to lose electrons (and thus, corrode).
- Shifting the Potential: CP shifts the metal’s electrical potential to a more negative value. This makes it less likely to act as an anode.
- The Role of the Electrolyte: The electrolyte (soil, water, etc.) is still present, but the CP system changes the electrochemical reactions happening within it.
| Aspect | Without CP | With CP |
|---|---|---|
| Metal Potential | More positive (anodic) | More negative (cathodic) |
| Electron Flow | Metal loses electrons (oxidation) | Metal gains electrons (from external source) |
| Corrosion Reaction | Favored | Suppressed |
| Rust Formation | Occurs | Prevented |
What are the Pros and Cons of Cathodic Protection?
Unsure if cathodic protection is the right solution for your corrosion problems? It is needed to weigh the benefits against the potential drawbacks.
The main pros of CP include effective corrosion prevention, and a longer structure lifespan. The cons include ongoing maintenance, and the risk of interference with other systems.
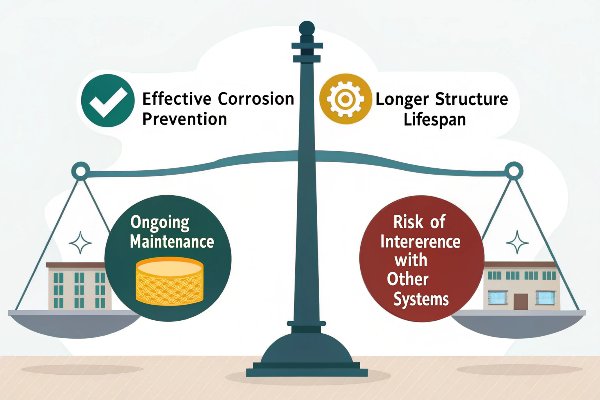
A Detailed Examination
Let’s break down the advantages and disadvantages more systematically:
-
Advantages (Pros):
- Effectiveness: It can significantly reduce corrosion rates, sometimes by over 99%.
- Lifespan Extension6: It dramatically extends the useful life of metal structures.
- Versatility: It can be used on a wide range of buried or submerged structures.
- Cost-Effectiveness: While there are upfront costs, it can prevent far more expensive repairs or replacements down the line.
-
Disadvantages (Cons):
- Monitoring: Regular monitoring and maintenance.
- Interference: Impressed current systems can sometimes interfere with other nearby buried metal structures.
- Overprotection: Applying too much current can, in some cases, lead to other problems like hydrogen embrittlement of certain metals.
| Factor | Pro | Con |
|---|---|---|
| Effectiveness | Highly effective at reducing corrosion. | |
| Lifespan | Extends the life of structures significantly. | |
| Cost | Can be more cost-effective than repairs/replacement in the long run. | Requires initial investment and ongoing maintenance costs. |
| Versatility | Applicable to various structures and environments. | |
| Maintenance | Requires regular monitoring and adjustments. | |
| Interference | Impressed current systems can interfere with other structures. | |
| Overprotection Risk | Can cause issues like hydrogen embrittlement if not properly controlled. |
What Type of Piping Requires Cathodic Protection?
Wondering if the pipes in your specific project or industry need this kind of protection? Making the wrong choice is a risk.
Piping that carries potentially corrosive materials7, or is buried in corrosive soil, often requires cathodic protection8. This commonly includes pipelines for oil, gas, and water.

A Closer Look at Specific Applications
Let’s get more specific about where CP is essential:
- Underground Pipelines:
- Oil and Gas Pipelines: These are almost always cathodically protected due to the high risk of leaks.
- Water and Wastewater Pipes: CP is increasingly used, especially for larger diameter pipes and those in aggressive soils.
- Distribution Networks: Smaller diameter pipes in distribution systems may also use CP, depending on local regulations and soil conditions.
- Other Buried Structures:
- Storage Tank Bottoms: The part of a storage tank that rests on the ground is highly susceptible to corrosion.
- Reinforced Concrete Structures: CP can be used to protect the steel rebar within concrete, especially in marine environments.
| Piping Type | Material | Environment | CP Requirement |
|---|---|---|---|
| Oil & Gas Pipeline | Steel | Buried (various soils) | Typically Yes |
| Water Pipeline (Large Diameter) | Steel, Ductile Iron | Buried (various soils) | Often Yes |
| Water Distribution (Small) | Various | Buried (less aggressive) | Sometimes |
| Chemical Process Piping | Various Alloys | Above ground, potentially | Depends |
Conclusion
Cathodic protection9 is a powerful tool. It safeguards vital infrastructure10, and prevents costly damage11. While it requires some care, the benefits far outweigh the drawbacks!
-
This resource will provide insights into the corrosion of metal structures and effective prevention methods, enhancing your knowledge. ↩
-
Explore this link to understand the principles and applications of cathodic protection in preventing corrosion effectively. ↩
-
Understanding the causes of corrosion can help you implement better protection strategies for your metal assets. ↩
-
Learn about the causes of rust and effective prevention methods to protect your metal assets. This resource offers valuable information. ↩
-
Delve into the science of rust formation and prevention through electrochemical processes. This link provides essential knowledge for better protection. ↩
-
Exploring lifespan extension benefits can reveal how to maximize the durability of your investments. ↩
-
Learn about corrosive materials and their impact on piping systems to make informed decisions for your projects. This resource provides essential information. ↩
-
Understanding cathodic protection is crucial for preventing corrosion in pipelines, ensuring safety and longevity. Explore this resource for in-depth insights. ↩
-
Explore this link to understand the principles and applications of cathodic protection in safeguarding infrastructure. ↩
-
Learn how cathodic protection plays a crucial role in protecting essential structures from corrosion and damage. ↩
-
Discover the economic advantages of implementing cathodic protection to avoid expensive repairs and maintenance. ↩





