Struggling to decide between electrowinning and electrorefining for your copper extraction needs? Feeling lost in the technical jargon and complex process comparisons?
Electrowinning and electrorefining are both electrochemical processes used to extract copper, but they differ in their starting materials and applications. Electrowinning extracts copper from a solution containing dissolved copper, while electrorefining purifies impure copper anodes.
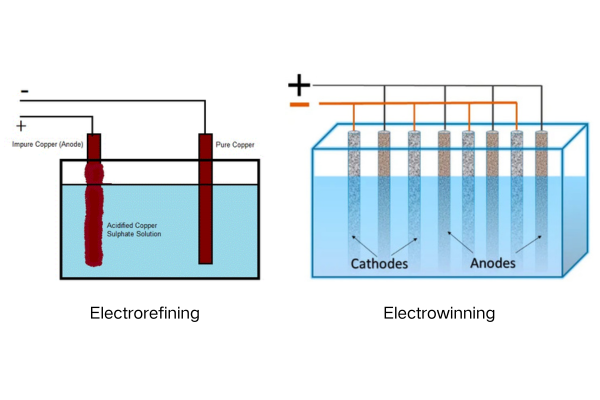
This article breaks down the critical differences, focusing on a key component: the anode. We’ll explore why titanium anodes are revolutionizing both processes, leaving traditional lead anodes behind. Let’s dive in!
Why use titanium anodes instead of lead alloy in copper electrowinning/electrorefining?
Worried about the environmental impact1 and long-term costs of lead anodes? Thinking there must be a better, more sustainable solution for copper extraction?
Titanium anodes offer superior longevity, corrosion resistance, and eliminate lead contamination2 risks compared to lead alloy anodes, leading to a lower total cost of ownership despite a higher initial investment.

Diving Deeper into Anode Choices
The choice between titanium and lead anodes significantly impacts the efficiency, environmental footprint, and overall cost of copper electrowinning and electrorefining. Here’s a detailed breakdown:
| Feature | Titanium Anodes | Lead Alloy Anodes |
|---|---|---|
| Lifespan | Significantly longer (years) | Shorter (months to a few years) |
| Corrosion | Highly resistant | Prone to corrosion and passivation |
| Environmental Impact | Minimal, no lead contamination | Lead contamination risk, disposal concerns |
| Energy Efficiency | Lower overpotential, reduced energy consumption | Higher overpotential, increased energy consumption |
| Maintenance | Lower frequency of replacement and maintenance | Higher frequency of replacement and maintenance |
| Initial Cost | Higher | Lower |
| Total Cost | Lower over the anode’s lifespan | Higher, due to replacement, disposal, and downtime |
The shift towards titanium anodes is also driven by increasingly stringent environmental regulations restricting lead usage. This, along with the substantial long term savings help me make a clear decision.
How to extend the lifespan of titanium anodes in high-acid copper electrolytes?
Concerned about the lifespan of titanium anodes in the harsh, high-acid environment of copper electrolytes? Wondering how to minimize downtime and maximize your investment?
You can extend titanium anode lifespan through specialized acid-resistant coatings (like ruthenium-iridium), precise electrolyte pH and temperature control, and monitoring for signs of anode passivation.

Optimizing for Longevity
Maintaining the integrity of titanium anodes in aggressive electrolytes requires a multi-pronged approach:
- Coating Technology:
- Ruthenium-Iridium Oxide3: This mixed metal oxide (MMO) coating is renowned for its exceptional resistance to acidic corrosion and its catalytic activity in oxygen evolution reactions.
- Coating Thickness and Uniformity: Precise control over coating thickness and uniformity is crucial for ensuring consistent performance and preventing premature failure.
- Electrolyte Management:
- pH Control4: Maintaining the optimal pH range (typically between 1.5 and 2.0 for copper electrowinning) is critical. Deviations can accelerate corrosion.
- Temperature Control: Elevated temperatures can increase corrosion rates. Maintaining a stable temperature within the recommended range (usually 40-60°C) is essential.
- Regular Monitoring: Frequent analysis of the electrolyte’s composition helps identify potential issues before they escalate.
- Passivation Prevention5:
- Current Density Threshold: Avoid exceeding the recommended current density for the specific anode coating. High current densities can lead to accelerated passivation.
- I found that Early Detection: Regularly inspect anodes for signs of passivation, such as increased cell voltage or uneven current distribution.
What are the best coating materials for titanium anodes in electrorefining pure copper?
Looking for the optimal coating to achieve ultra-high purity copper in your electrorefining process? Balancing cost with performance is a key challenge, right?
For electrorefining pure copper, Iridium-Tantalum (Ir-Ta)6 coatings offer an excellent balance of conductivity, catalytic efficiency, and corrosion resistance, although other options like Tin-Antimony (Sn-Sb)7 exist.

Exploring Coating Options
The selection of coating materials significantly impacts the quality and efficiency of the electrorefining process. Let’s look deeper:
- Iridium-Tantalum (Ir-Ta):
- Excellent Conductivity: Ensures efficient electron transfer.
- High Catalytic Activity: Promotes the desired electrochemical reactions.
- Superior Corrosion Resistance: Withstands the harsh conditions of the electrolyte.
- Relatively High Cost: Due to the use of precious metals.
- Tin-Antimony (Sn-Sb):
- Lower Cost Alternative: Compared to Ir-Ta.
- Good Corrosion Resistance: In certain electrolyte compositions.
- Lower Catalytic Activity: May result in slightly lower efficiency.
- Advanced Coating Technologies8
Gradient Coatings: These coatings have a composition that varies across their thickness, optimizing performance at different layers.
Nanostructured Coatings: Utilizing nanotechnology to create coatings with enhanced surface area and catalytic activity.
Can titanium anodes handle impurities like iron and arsenic in electrowinning solutions?
Worried about how impurities in your electrowinning solution might affect the performance and lifespan of your titanium anodes? Concerned about potential cathode contamination9?
Titanium anodes exhibit good corrosion resistance to common impurities like iron and arsenic, but high concentrations may require pretreatment of the electrolyte to ensure optimal performance and prevent cathode copper contamination.
Managing Impurities
The presence of impurities in the electrowinning electrolyte can pose several challenges:
- Anode Corrosion: While titanium is generally resistant, excessive levels of certain impurities, especially at high temperatures and current densities, can accelerate corrosion.
- Cathode Contamination: Impurities can co-deposit with copper on the cathode, reducing the purity of the final product.
- Electrolyte Degradation: Some impurities can react with the electrolyte, leading to undesirable byproducts and reduced efficiency.
- Mitigation Strategies
- Pretreatment: Employing techniques like ion exchange or solvent extraction to remove impurities before the electrowinning process.
- Electrolyte Bleed: Regularly removing a portion of the electrolyte and replacing it with fresh solution to control impurity buildup.
- Careful Anode Selection: Choosing anode coatings specifically designed for resistance to the particular impurities present.
- Pretreatment: Employing techniques like ion exchange or solvent extraction to remove impurities before the electrowinning process.
- Data Insights: Based on my experience, experimental data demonstrates that titanium anodes can maintain acceptable performance in electrolytes containing moderate levels of iron (Fe) and arsenic (As), up to certain concentration limits (which vary depending on specific conditions). Beyond these limits, pretreatment becomes essential.
Are titanium anodes suitable for both small-scale and industrial copper electrowinning plants?
Wondering if titanium anodes are a viable option for your small-scale operation? Or, are you unsure if they can scale up to meet the demands of a large industrial plant?
Titanium anodes are suitable for both small-scale and industrial copper electrowinning10 plants, offering modular designs11 for smaller operations and robust configurations for large-scale facilities.

Scaling with Titanium Anodes
The adaptability of titanium anodes makes them a versatile choice for various scales of operation:
- Small-Scale Applications:
- Modular Electrolytic Cells: These designs allow for easy expansion or reduction of capacity as needed.
- Simplified Maintenance: Smaller anodes are easier to handle and replace.
- Lower Initial Investment: Smaller systems require a smaller upfront investment in anodes.
- Industrial-Scale Applications:
- Robust Anode Configurations: Larger, more durable anodes are designed for continuous operation in demanding environments.
- Automated Systems: Integration with automated control systems for optimized performance and reduced labor costs.
- Long-Term Cost Savings: The extended lifespan and reduced maintenance of titanium anodes translate to significant cost savings over time.
- Performance Comparison
- I believe that testing has shown that titanium anodes exhibit minimal performance degradation when scaled from a 10L laboratory setup to a 1000m³ industrial cell, provided that proper design and operating parameters are maintained.
Conclusion
Titanium anodes are transforming copper electrowinning and electrorefining, offering superior performance, environmental benefits, and long-term cost savings compared to traditional lead anodes, across all scales of operation.
-
Learn about the environmental consequences of lead alloy anodes to understand the need for sustainable practices in copper production. ↩
-
Understanding lead contamination risks can help you appreciate the importance of choosing safer alternatives in copper extraction. ↩
-
Explore the advantages of Ruthenium-Iridium Oxide coatings to enhance the durability and performance of titanium anodes in harsh environments. ↩
-
Understanding the impact of pH control on titanium anodes can help you optimize their lifespan and performance in copper electrolytes. ↩
-
Learn about effective strategies to prevent passivation in titanium anodes, ensuring their longevity and efficiency in electrochemical processes. ↩
-
Explore the advantages of Ir-Ta coatings for enhanced conductivity and corrosion resistance in copper electrorefining. ↩
-
Learn about the cost-effectiveness and performance of Sn-Sb coatings as an alternative to Ir-Ta in electrorefining. ↩
-
Discover cutting-edge coating technologies that can improve efficiency and purity in electrorefining processes. ↩
-
Learn about the factors leading to cathode contamination and how to mitigate them for better purity in your final product. ↩
-
Learn about industrial copper electrowinning processes and how they can benefit from advanced technologies like titanium anodes. ↩
-
Discover how modular designs can enhance flexibility and efficiency in electrolysis systems, especially for small-scale operations. ↩





