Struggling to understand the nuances of electrowinning and electrorefining? Feeling lost in a sea of technical jargon and complex processes?
Electrowinning and electrorefining are both electrochemical processes used to extract or purify metals, but they differ in their starting materials and applications. Electrowinning recovers metals from solutions, while electrorefining purifies impure metal anodes.

This article will clarify those questions for you. Let’s Dive in.
What’s the Difference Between Electrowinning and Electrorefining?
Confused about which process, electrowinning or electrorefining, is right for your needs? Are you looking for the most cost-effective and efficient way to obtain high-purity metals1?
Electrowinning2 extracts metals from a solution, typically after leaching from ore. Electrorefining3, on the other hand, uses an impure metal anode to produce a high-purity metal cathode.
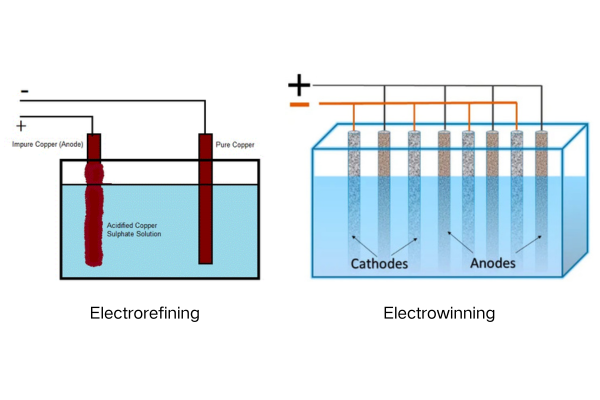
Diving Deeper: Understanding the Core Distinctions
The core difference lies in the source material and the desired outcome. Think of it this way: electrowinning is like extracting gold from river water (low concentration), while electrorefining is like purifying a gold bar to make it even more valuable (high concentration).
| Feature | Electrowinning | Electrorefining |
|---|---|---|
| Process Flow | Leaching -> Solution Purification -> Electrowinning | Dissolution of Impure Anode -> Electrorefining |
| Metal Purity | Lower (directly from ore leachate) | Higher (refining already impure metal) |
| Cost | Generally lower (for low-grade ores) | Can be higher (depends on impurity levels) |
| Application | Primary metal extraction from ores | Refining of impure metals, recycling |
Example:
I witnessed this firsthand. A copper mine, used electrowinning to extract copper directly from a low-grade ore solution. A scrap metal recycling facility, however, uses electrorefining to purify the copper from discarded electronics, achieving a much higher purity level.
Which Metals Can Be Extracted Using Electrowinning?
Are you wondering if electrowinning is suitable for the specific metals you’re working with? Are you involved in the burgeoning field of new energy metals and curious about their extraction methods?
Commonly electrowon metals include copper, zinc, gold, silver, nickel, and cobalt. Emerging applications include lithium-ion battery recycling4, recovering valuable metals from spent batteries.

Diving Deeper: Beyond the Common Metals
While the traditional metals mentioned above dominate electrowinning applications5, the technology is constantly evolving. The rise of electric vehicles and the increasing demand for lithium-ion batteries have spurred research and development into electrowinning for battery recycling.
| Metal | Traditional Application | Emerging Application | Commercialization Threshold |
|---|---|---|---|
| Copper | Primary extraction from ores | Battery recycling (minor) | High (20% of global production) |
| Zinc | Primary extraction | – | High |
| Gold & Silver | Primary extraction, byproduct | – | High |
| Nickel | Primary extraction | Battery recycling (growing) | Medium |
| Cobalt | Byproduct of copper/nickel | Battery recycling (significant) | Medium |
| Lithium | – | Battery recycling (early stage) | Low, but rapidly increasing |
I believe the commercial viability of electrowinning6 for a specific metal often depends on factors like ore grade, energy costs, and environmental regulations. Copper electrowinning, for example, is widely adopted due to its relatively low cost and environmental advantages over traditional smelting.
Is Electrowinning Environmentally Friendly?
Are you seeking metal extraction methods that align with ESG (Environmental, Social, and Governance) standards? Are you looking for low-pollution alternatives to traditional, high-emission processes?
Compared to pyrometallurgical processes (smelting), electrowinning is generally considered more environmentally friendly. It avoids high-temperature operations and associated sulfur dioxide emissions.

Diving Deeper: Balancing Benefits and Risks
While electrowinning offers a "greener" approach, it’s crucial to acknowledge potential environmental risks. The process typically involves acidic solutions7, which, if not handled properly, can contaminate water sources.
| Environmental Aspect | Benefit | Potential Risk | Mitigation Strategy |
|---|---|---|---|
| Air Emissions | No sulfur dioxide emissions | – | – |
| Water Usage | Can be lower than some smelting processes | Acidic solution leakage/discharge | Closed-loop water treatment system |
| Energy Consumption | Can be lower than smelting | Depends on electricity source | Utilize renewable energy sources8 |
| Waste Generation | Less solid waste than smelting | Spent electrolyte disposal | Proper treatment and disposal/recycling |
| Overall Impact | Generally lower | Site-specific factors must be considered | Comprehensive environmental impact assessment |
My friend told me his mining company, switched to electrowinning and reduced its carbon footprint by approximately 30%. This demonstrates the potential for significant environmental improvements. However, responsible operation and stringent environmental controls are paramount.
How Much Does an Electrowinning Plant Cost?
Are you a small or medium-sized mining company evaluating the economic feasibility of electrowinning? Are you seeking a detailed breakdown of costs to determine the return on investment (ROI)9?
The cost of an electrowinning plant10 varies greatly depending on capacity, technology, and location. Major cost components11 include the electrolyzer, electrodes, power supply, infrastructure, and labor.

Diving Deeper: Itemized Cost Analysis
A detailed cost analysis is crucial for any potential investor. Let’s break down the major components:
| Cost Component | Description | Estimated Percentage of Total Cost |
|---|---|---|
| Electrolyzer | The core of the plant, where electrolysis occurs | 30-40% |
| Electrodes (Anode/Cathode) | Materials can vary (lead, stainless steel, etc.) | 15-25% |
| Power Supply | Rectifiers, transformers, etc. | 10-20% |
| Infrastructure | Building, tanks, piping, etc. | 10-20% |
| Labor | Operating and maintenance personnel | 5-10% |
| Other | Reagents, permitting, etc. | 5-10% |
For a hypothetical 10-ton-per-day copper electrowinning plant, the initial capital investment could range from $1 million to $3 million, depending on the specific technology and location. Operating costs, primarily electricity, would be ongoing.
Why Is My Electrowinning Efficiency Low?
Are you experiencing low current efficiency and slow metal deposition rates in your electrowinning operation? Are you struggling to pinpoint the root cause of these inefficiencies?
Low electrowinning efficiency can stem from various factors, including insufficient ion concentration in the electrolyte, temperature fluctuations, electrode corrosion or passivation, and impurities in the solution.
Diving Deeper: Troubleshooting Common Issues
Troubleshooting low efficiency requires a systematic approach. Here are some common culprits and potential solutions:
| Problem | Possible Cause(s) | Solution(s) |
|---|---|---|
| Low Current Efficiency12 | Low metal ion concentration, high impurity levels | Optimize electrolyte composition, improve solution purification |
| Slow Deposition Rate13 | Low temperature, low current density | Increase temperature (within optimal range), adjust current density |
| Electrode Corrosion/Passivation14 | Aggressive electrolyte, improper electrode material | Use corrosion-resistant electrodes, adjust electrolyte pH, add inhibitors |
| Uneven Deposition | Poor electrolyte circulation, non-uniform current density | Improve agitation, optimize electrode spacing and geometry |
| High Energy Consumption | High resistance, poor electrical connections | Check connections, use appropriate electrolyte additives, optimize cell design |
I’ve learned that Addressing these issues often involves a combination of optimizing the electrolyte formula, implementing automatic temperature control, and ensuring proper electrode maintenance. Regular monitoring and analysis are key to maintaining high efficiency.
Can Electrowinning Remove Impurities from Metals?
Worried about meeting stringent purity standards for your metals? Wondering if electrowinning can truly deliver the high-quality results you need?
Yes, electrowinning can effectively remove impurities from metals through a process called selective deposition15. However, pretreatment of the solution and the use of ion exchange resins16 may be necessary to achieve ultra-high purity levels.
Understanding the Mechanism & Achieving Ultra-High Purity
Electrowinning selectively deposits the desired metal onto the cathode while leaving impurities in the solution. However, achieving exceptionally high purity (like 99.99% for copper) often requires additional steps.
Here’s a breakdown:
1. Selective Deposition
During electrowinning, a current is passed through an electrolyte solution containing dissolved metal ions. The desired metal ions are reduced at the cathode, forming a pure metal deposit. Impurities with different electrochemical properties remain in the solution.
2. Pretreatment of the Solution
Before electrowinning, the electrolyte solution often undergoes pretreatment. This may involve:
- Filtration: Removing suspended solids.
- Chemical Precipitation: Selectively precipitating certain impurities.
- Solvent Extraction: Separating metal ions based on their solubility in different solvents.
3. Ion Exchange Resins
For ultra-high purity, ion exchange resins can be employed.
These resins selectively bind to and remove trace impurities from the electrolyte solution, further enhancing the purity of the final metal product.
4. Electrorefining
Electrorefining is usually more effective than electrowinning for high purity.
| Feature | Electrowinning | Electrorefining |
|---|---|---|
| Purity Limit | Typically up to 99.9% | Can achieve >99.99% purity |
| Anode Material | Insoluble anode | Impure metal anode |
| Main Purpose | Metal extraction | Metal purification |
| Example | Copper from leach solution | Copper refining from blister copper |
By combining these techniques, electrowinning can achieve impressive levels of metal purity, making it a crucial process in various industries.
What Electrodes are Best for Electrowinning?
Choosing the right electrode material can be a balancing act. Are you sacrificing longevity for cost, or vice versa? What material truly delivers the best performance for your specific needs?
The "best" electrode for electrowinning depends on the specific application and electrolyte composition. Common choices include titanium coated with iridium, stainless steel, and graphite, each with its own advantages and disadvantages regarding cost, lifespan, and conductivity.
A Deep Dive into Electrode Materials
Let’s compare the most common electrode materials:
1. Titanium Coated with Iridium17 (Dimensionally Stable Anodes – DSAs)
These anodes consist of a titanium substrate coated with a thin layer of iridium oxide.
- Pros: Excellent corrosion resistance (especially in highly acidic environments), high conductivity, long lifespan.
- Cons: Higher initial cost compared to other options.
- Best for: High-acid environments, applications requiring long electrode life.
2. Stainless Steel18
A common and relatively inexpensive option.
- Pros: Good corrosion resistance in many electrolytes, lower cost than titanium-based anodes.
- Cons: Shorter lifespan than DSAs, especially in highly corrosive environments; may contaminate the electrolyte with iron.
- Best for: Less aggressive electrolytes, applications where cost is a primary concern.
3. Graphite19
A non-metallic option with good conductivity.
- Pros: Relatively inexpensive, good conductivity.
- Cons: Can be brittle and prone to erosion, shorter lifespan, may introduce carbon particles into the electrolyte.
- Best for: Specific applications where carbon contamination is not a concern, cost-sensitive operations.
Comparison Table:
| Material | Corrosion Resistance | Conductivity | Unit Price | Lifespan | Best For |
|---|---|---|---|---|---|
| Titanium (IrO2 coated) | Excellent | Excellent | High | Long | High-acid environments, long-term use |
| Stainless Steel | Good | Good | Medium | Moderate | Less aggressive electrolytes, moderate cost |
| Graphite | Moderate | Good | Low | Short | Specific applications, low cost |
Choosing the optimal electrode requires careful consideration of the electrolyte chemistry, operating conditions, and budget constraints. I once had to replace stainless steel cathodes every few months. It was a constant headache!
How to Reduce Energy Consumption in Electrowinning?
Is the high cost of electricity eating into your profits? Are you looking for ways to make your electrowinning process more sustainable and cost-effective?
Energy consumption20, primarily electricity, is a significant cost factor in electrowinning, often representing 40-60% of operating expenses. Reducing energy use can be achieved by optimizing current density, employing catalytic anodes21, and implementing waste heat recovery systems22.
Strategies for Energy Efficiency
Here’s a deeper look at the three major strategies:
1. Optimizing Current Density
Current density is the amount of current per unit area of the electrode.
- Lower Current Density: Generally leads to lower energy consumption per unit of metal produced, but also slower production rates.
- Higher Current Density: Increases production rates, but can lead to higher energy consumption and potential side reactions.
- Optimal Current Density: Finding the sweet spot that balances energy efficiency and production rate is crucial. This can be achieved through careful experimentation and process control.
- Tip: Monitor cell voltage and current efficiency regularly.
2. Employing Catalytic Anodes (e.g., Mixed Metal Oxide Coatings)
These anodes reduce the overpotential required for the electrochemical reactions, thus lowering energy consumption.
- Mechanism: Catalytic coatings lower the activation energy for the desired reactions, making them proceed more easily.
- Example: Certain mixed metal oxide coatings can reduce energy consumption by up to 15% compared to traditional anodes.
- Note: Consider long-term cost savings vs initial investiment.
3. Implementing Waste Heat Recovery Systems
Electrowinning processes often generate significant heat.
- Heat Exchangers: Can be used to preheat the electrolyte solution, reducing the energy required to maintain the operating temperature.
- Other Applications: Recovered heat can be used for other processes within the plant, further improving overall energy efficiency.
- Example: A facility used waste heat for space heating, saving $200,000 per year in utility bills.
By implementing these strategies, significant reductions in energy consumption and operating costs can be achieved, making electrowinning a more sustainable and economically viable process.
Can Electrowinning Recycle Precious Metals from E-waste?
Concerned about the growing mountains of electronic waste? Curious about the potential of electrowinning to recover valuable resources from discarded devices?
Yes, electrowinning can play a crucial role in recycling precious metals like gold and palladium from e-waste. This involves a process of leaching the metals from shredded electronic components followed by electrolytic extraction. However, the complex composition of e-waste presents significant separation challenges.
The E-waste Recycling Process with Electrowinning
Here’s a detailed look at the process:
1. Collection and Dismantling
E-waste is collected and manually dismantled to separate components like printed circuit boards (PCBs), which contain the highest concentrations of precious metals.
2. Shredding and Pretreatment
PCBs are shredded into small pieces to increase the surface area for leaching. Pretreatment may involve physical separation techniques like magnetic separation to remove ferrous metals.
3. Leaching
The shredded material is treated with a leaching solution (e.g., cyanide, aqua regia, or thiosulfate) to dissolve the precious metals into solution.
4. Electrowinning
The precious metal-containing solution is then subjected to electrowinning.
- Gold and Palladium Recovery: Gold and palladium are selectively deposited onto the cathode, while other metals may remain in the solution or be recovered separately.
- Challenge: The complex mixture of metals in e-waste requires careful control of the leaching and electrowinning parameters to ensure selective recovery.
5. Refining
The recovered metals may undergo further refining to achieve the desired purity levels.
Success Stories: Some companies have achieved impressive recycling rates, with one facility reporting a 95% recovery rate for gold from e-waste using a combination of leaching and electrowinning.
What’s the Future of Electrowinning Technology?
Are you wondering what advancements are on the horizon for electrowinning? Are you looking to invest in a technology that’s poised for growth and innovation?
The future of electrowinning technology is focused on three major trends: AI-driven optimization23, the use of ionic liquid electrolytes, and the development of modular electrowinning equipment. These innovations promise to enhance efficiency, reduce environmental impact, and expand the applications of electrowinning.
1. AI-Driven Optimization
Artificial intelligence (AI) and machine learning are being increasingly integrated into electrowinning processes.
* **Dynamic Parameter Adjustment:** AI algorithms can analyze real-time data from sensors monitoring various parameters (temperature, current density, electrolyte composition) and automatically adjust operating conditions to optimize performance.
* **Predictive Maintenance:** AI can predict equipment failures and schedule maintenance proactively, minimizing downtime and maximizing efficiency.- Example: Real-time adjustments to current density based on electrolyte analysis.
2. Ionic Liquid Electrolytes24
Traditional electrowinning often uses aqueous electrolytes, which can have limitations in terms of operating temperature and environmental impact.
- Advantages of Ionic Liquids:
- Wider Electrochemical Window: Allowing for the deposition of metals that are difficult to extract from aqueous solutions.
- Higher Thermal Stability: Enabling operation at higher temperatures, potentially improving reaction kinetics.
- Lower Vapor Pressure: Reducing the risk of harmful emissions.
- Wider Electrochemical Window: Allowing for the deposition of metals that are difficult to extract from aqueous solutions.
- Challenges: Higher cost and viscosity compared to aqueous electrolytes.
- Note: Research is ongoing to develop more cost-effective and less viscous ionic liquids.
3. Modular Electrowinning Equipment25
Modular systems offer flexibility and scalability.
- Benefits:
- Scalability: Easily add or remove modules to adjust production capacity as needed.
- Flexibility: Adapt to different feed materials and process requirements.
- Reduced Footprint: Smaller, more compact units compared to traditional large-scale installations.
- Scalability: Easily add or remove modules to adjust production capacity as needed.
- Applications: Ideal for decentralized e-waste recycling, small-scale mining operations, and research and development.
Market Projections: Authoritative reports project significant growth in the electrowinning market, with one estimate reaching $XX billion by 2030, driven by increasing demand for metals, growing e-waste recycling initiatives, and advancements in technology.
Conclusion
Electrowinning is a powerful and evolving technology with a wide range of applications. From achieving high metal purity to recovering valuable resources from e-waste, electrowinning continues to play a crucial role in various industries.
-
Learn about the most efficient methods for obtaining high-purity metals, crucial for various industrial applications. ↩
-
Explore this link to gain a deeper understanding of electrowinning, its processes, and applications in metal extraction. ↩
-
Discover the benefits and processes of electrorefining, which can enhance your knowledge of metal purification techniques. ↩
-
Explore this link to understand the innovative processes behind lithium-ion battery recycling and its significance in metal recovery. ↩
-
Discover the latest advancements in electrowinning applications and how they are transforming metal extraction processes. ↩
-
Learn about the key factors that determine the commercial viability of electrowinning, crucial for understanding its economic impact. ↩
-
Learn about the risks associated with acidic solutions in electrowinning and how to mitigate them effectively. ↩
-
Discover how renewable energy can enhance the sustainability of electrowinning and reduce its carbon footprint. ↩
-
Learn how to effectively calculate ROI for electrowinning projects to assess their financial viability. ↩
-
Explore this link to understand the financial implications and factors affecting the cost of electrowinning plants. ↩
-
This resource will provide a detailed breakdown of the cost components, helping you make informed investment decisions. ↩
-
Understanding the causes of low current efficiency can help you implement effective solutions to improve your electrowinning process. ↩
-
Exploring factors affecting deposition rates can lead to improved metal recovery and operational efficiency in your electrowinning process. ↩
-
Preventing electrode corrosion is crucial for maintaining efficiency and prolonging the lifespan of your equipment. ↩
-
Explore this link to understand how selective deposition enhances metal purity in electrowinning processes. ↩
-
Learn about the role of ion exchange resins in achieving ultra-high purity in metals through electrowinning. ↩
-
Explore the advantages of Titanium Coated with Iridium, especially in high-acid environments, to enhance your electrowinning process. ↩
-
Learn why Stainless Steel is favored for its cost-effectiveness and corrosion resistance in less aggressive environments. ↩
-
Discover the unique properties of Graphite electrodes and their suitability for specific applications in electrowinning. ↩
-
Explore this resource to discover effective strategies for minimizing energy costs in electrowinning, enhancing sustainability and profitability. ↩
-
Learn how catalytic anodes can significantly lower energy consumption and boost your electrowinning process efficiency. ↩
-
Discover the advantages of waste heat recovery systems and how they can lead to substantial cost savings in your operations. ↩
-
Explore how AI is revolutionizing electrowinning processes, enhancing efficiency and reducing costs. ↩
-
Learn about the advantages of ionic liquids in electrowinning and their potential to improve metal extraction. ↩
-
Discover how modular systems are transforming electrowinning, making it more adaptable and efficient for various applications. ↩





