Struggling to understand the nuances between PbO and PbO2 in your electrochemical setups? You’re not alone, it’s a common point of confusion.
PbO (lead monoxide) and PbO2 (lead dioxide) are distinct compounds with different oxidation states, structures, and properties, leading to varied uses. PbO often serves as a precursor or negative electrode material, while PbO2, a strong oxidizer, is crucial in positive electrodes, like those in lead-acid batteries.

But that’s just scratching the surface. Let’s dive into a detailed comparison to truly understand their roles and applications, and I promise you will find the differences between them.
What is the difference between PbO and PbO2?
Confused about which lead oxide to use where? It’s easy to get them mixed up, but choosing the wrong one could ruin your experiment or process.
The core difference lies in their oxidation state1: PbO has lead in the +2 state, while PbO2 has lead in the +4 state. This seemingly small change results in different colors (yellow/brown vs. dark brown) and crystal structures, profoundly impacting their chemical behavior2.
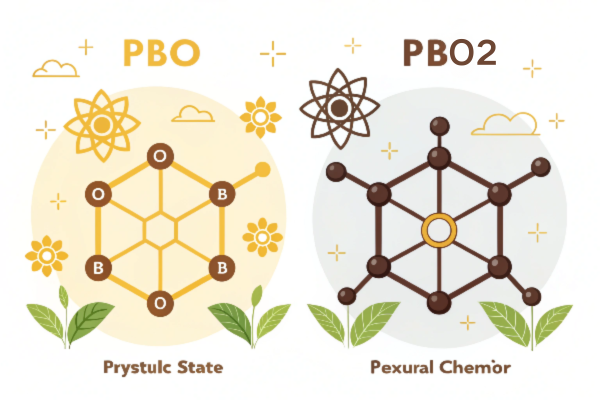
Diving Deeper: PbO vs. PbO2 – A Structural and Application Perspective
The differences extend beyond basic chemistry. Let’s examine how these differences impact their applications. I am goint to take the battery industry3 as an example:
| Feature | PbO (Lead Monoxide) | PbO2 (Lead Dioxide) |
|---|---|---|
| Oxidation State | +2 | +4 |
| Color | Yellow to reddish-yellow or brown | Dark brown to black |
| Structure | Litharge (tetragonal), Massicot (orthorhombic) | Various polymorphs, often β-PbO2 (rutile-like) |
| Battery Role | Negative Electrode (often as a precursor) | Positive Electrode (active material) |
| Other Uses | Pigments, ceramics, glass manufacturing | Oxidant in chemical synthesis, wastewater treatment |
Think of it like this, in a lead-acid battery, PbO is like the starting material that gets transformed during charging, whereas PbO2 is the active participant in the discharge process, providing the oxidizing power. It will be a personal disaster if you use them wrongly.
What is the role of PbO2?
Are you aware of the hidden potential and risks associated with PbO2 in various industries? Ignoring them could lead to missed opportunities or even safety hazards.
PbO2’s primary role is as a powerful oxidizing agent4. In lead-acid batteries5, it’s the active material of the positive electrode, driving the electrochemical reactions. It also finds use in wastewater treatment6 for oxidizing pollutants and in some chemical syntheses.
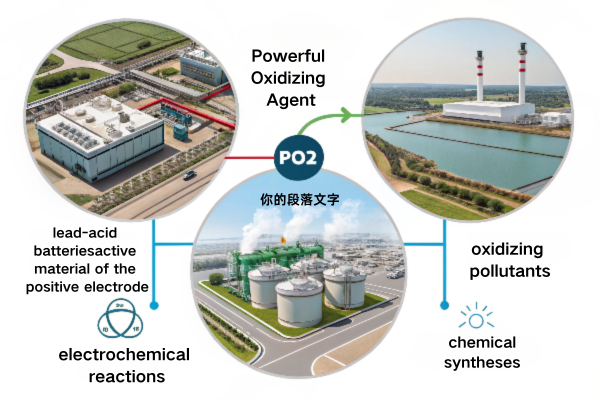
Diving Deeper: PbO2 – Beyond the Battery
PbO2’s oxidizing power isn’t limited to batteries. Let’s explore its broader impact:
- Lead-Acid Batteries: The heart of its application. PbO2 undergoes reduction during discharge, providing electrons.
- Wastewater Treatment: Its strong oxidizing ability helps break down organic pollutants, making water safer.
- Electrosynthesis: Used to produce various chemicals, taking taking advantage of it’s strong oxidizing power.
- Safety Considerations: Handling PbO2 requires caution due to its toxicity and the potential for lead exposure. Proper disposal of lead-containing waste is crucial to protect the environment.
It’s clear that PbO2 plays a significant, although sometimes overlooked, role in various industrial processes. Improper use could lead to severe environmental problems.
What are the properties of PbO2?
Considering using PbO2 in your next project, but unsure if it’s the right fit? You could be facing performance issues or unexpected reactions if you don’t understand its properties.
PbO2 is a dark brown, crystalline solid7 with a density of around 8.9 g/cm³. It’s practically insoluble in water but can react with acids and strong bases. Critically, it’s a powerful oxidizing agent8 and is relatively thermally stable9, although it decomposes at higher temperatures.
Diving Deeper: Unveiling the Nature of PbO2
Let’s delve into a more comprehensive look at PbO2’s properties:
| Property | Description |
|---|---|
| Physical State | Solid (crystalline) |
| Color | Dark brown to black |
| Density | ~8.9 g/cm³ |
| Solubility | Insoluble in water; soluble in strong acids and bases |
| Oxidizing Power | Strong oxidizer |
| Thermal Stability | Relatively stable at room temperature; decomposes at higher temperatures (>290°C) |
| Crystal structure | Exists in different polymorphs, the most important being α-PbO2 and β-PbO2. β-PbO2 has an orthorhombic crystal structure, and is similar to rutile (TiO2). |
Comparing PbO2 to other common oxides, like MnO2, reveals similarities in their oxidizing capabilities but differences in their stability and specific applications. Each oxide has its own niche.
Which is more stable, PbO or PbO2?
Concerned about the longevity and safety of your lead-based materials? Choosing the wrong form could lead to premature degradation or unexpected hazards.
At room temperature, PbO is generally more stable. PbO2, while relatively stable, tends to decompose at higher temperatures (above approximately 290°C), releasing oxygen and forming lower lead oxides.
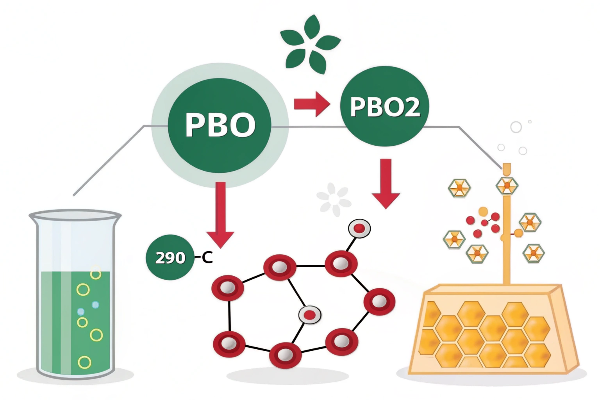
Diving Deeper: Stability Under Different Conditions
Thermodynamic stability is crucial, but practical stability depends on the environment. Let’s break it down:
- Room Temperature: PbO is favored. PbO2 can exist but is less thermodynamically stable.
- High Temperature: PbO remains stable to higher temperatures. PbO2 decomposes.
- Applications: This stability difference dictates usage. PbO2 is not suitable for high-temperature applications where its decomposition would be problematic. In contrast, in the lower working temperature battery, PbO2 is extensively used.
It’s important to note that the above is a thermodynamic analysis. There are many influence factors in practice.
What happens when PbO2 is heated?
Worried about the safety of heating lead dioxide? Ignoring the reaction could lead to hazardous fumes and process failures.
When PbO2 is heated above its decomposition temperature10 (around 290°C), it breaks down, releasing oxygen and forming lower lead oxides11, such as Pb3O4 (red lead) or eventually PbO.
Diving Deeper: The Decomposition Pathway
The heating process isn’t a single step. It’s a cascade:
- Initial Decomposition: PbO2 → Pb12O19
- Further Decomposition: Pb12O19 → Pb3O4 + O2↑
- Final Stage (high temperature): Pb3O4 → PbO + O2↑
The exact sequence and products depend on the temperature and heating rate. This reaction is crucial in some industrial processes, like lead recovery, but it also highlights the need for proper ventilation and safety measures due to the potential release of toxic lead compounds12. I once witnessed a lab accident caused by improper handling of heated lead oxides.
Conclusion
PbO and PbO2, while both lead oxides, have distinct properties and applications. Understanding these differences, from their oxidation states13 to their thermal stability14, is crucial for using them effectively and safely in various electrochemical contexts.
-
Understanding oxidation states is crucial for grasping chemical reactions and properties, making this resource invaluable for your studies. ↩
-
Delve into the factors affecting chemical behavior to better understand reactions involving lead oxides and their applications. ↩
-
Explore how these compounds are essential in battery technology, enhancing your knowledge of their practical applications. ↩
-
Understanding oxidizing agents can enhance safety and efficiency in various industries. Explore this link to learn more. ↩
-
Lead-acid batteries are crucial in energy storage. Discover their workings and applications to leverage their benefits. ↩
-
Effective wastewater treatment is vital for environmental safety. Learn about innovative methods using oxidizing agents. ↩
-
Learning about crystalline solids can enhance your understanding of PbO2’s structure and behavior in various conditions. ↩
-
Understanding the applications of powerful oxidizing agents can help you leverage PbO2 effectively in your projects. ↩
-
Exploring thermally stable materials can provide insights into how PbO2 compares and its potential uses in high-temperature applications. ↩
-
Understanding the decomposition temperature is crucial for safe handling and processing of PbO2 to prevent hazardous reactions. ↩
-
Exploring the properties of lead oxides can enhance your knowledge of their applications and safety measures in industrial processes. ↩
-
Learning about the dangers of toxic lead compounds can help you implement necessary safety precautions in your work environment. ↩
-
This resource will help you understand the importance of oxidation states in chemistry and their impact on compound behavior. ↩
-
Learn about thermal stability and its implications for material performance in different applications by visiting this link. ↩





