Struggling to grasp electrochemical concepts? Feeling lost in a sea of confusing terminology like "anode" and "cathode"? You’re not alone.
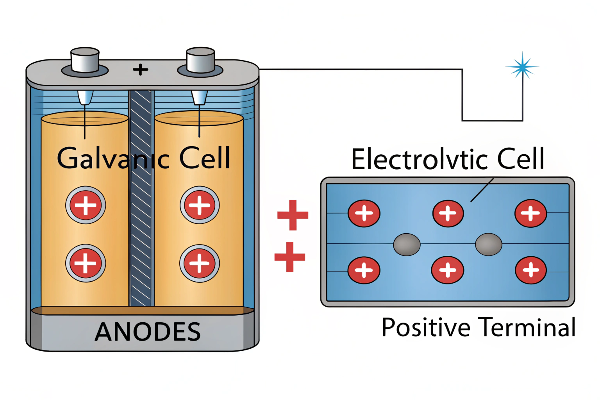
An anode is an electrode where oxidation occurs. In a galvanic cell (like a battery), it’s the negative terminal; in an electrolytic cell (like electroplating), it’s the positive terminal. Electrons flow from the anode in a galvanic cell and to the anode in an electrolytic cell.
But that’s just scratching the surface. Let’s dive deeper into the world of anodes, exploring their function, types, and real-world applications. I, Euros Yang, from Xuboti, will guide you through. Let’s continue!
What is an Anode Short Answer?
Having problems to understand what exactly anode is? The definition has too many professional words? I have been there.
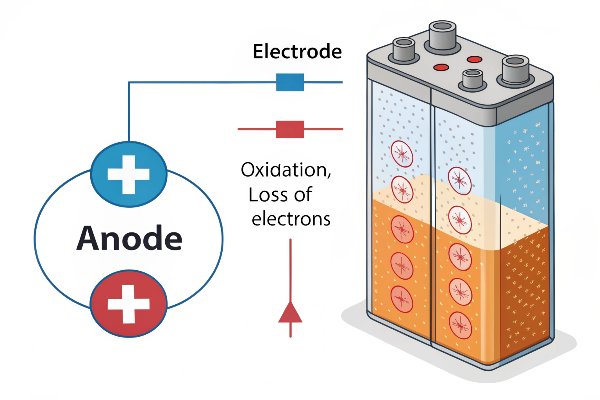
An anode is the electrode where oxidation (loss of electrons) takes place. Its polarity (+ or -) depends on the cell type (galvanic or electrolytic).
Diving Deeper into Anode Definitions
Let’s break down the core concept: oxidation1. Anodes are all about giving up electrons. Think of it like this:
| Feature | Description | Analogy |
|---|---|---|
| Oxidation | Loss of electrons by a substance. | A generous friend giving away gifts. |
| Electrode | A conductor through which electricity enters or leaves. | A doorway for electricity. |
| Polarity | Positive (+) or negative (-) charge. | North (+) or South (-) pole of a magnet. |
The definition can be confusing because the anode’s polarity changes depending on the context. In batteries (galvanic cells), the anode is negative because it supplies electrons to the external circuit. In electrolysis (electrolytic cells), the anode is positive because it receives electrons from the external power source. My factory primarily focuses on anodes for electrolytic applications, specifically titanium anodes for water treatment and electrolysis. We at www.xuboanode.com serve clients like Victor, a technical manager from Canada, who needs high-quality, yet cost-effective, titanium anodes.
Is Anode Usually Positive or Negative?
Confused about the anode’s charge? Is it positive or negative? It’s a common question, and the answer isn’t always straightforward.
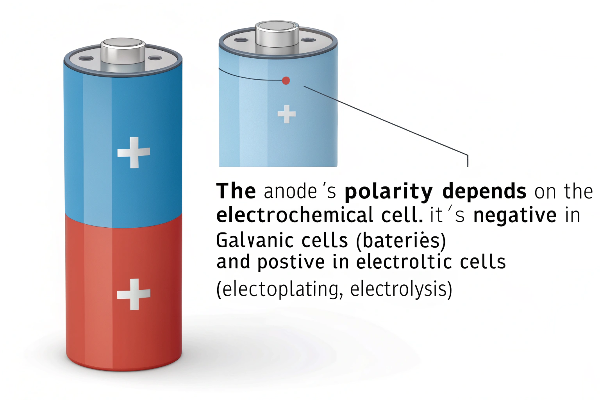
The anode’s polarity depends on the electrochemical cell. It’s negative in galvanic cells2 (batteries) and positive in electrolytic cells3 (electroplating, electrolysis).
Delving into Anode Polarity
The key is the direction of electron flow. This can change! Let us see:
| Cell Type | Anode Polarity | Electron Flow | Example |
|---|---|---|---|
| Galvanic (Battery) | Negative (-) | From anode to external circuit | Discharging battery |
| Electrolytic | Positive (+) | To anode from power source | Electroplating, electrolysis |
Think of a battery discharging: electrons flow from the negative anode to the positive cathode, powering a device. Now, think of electroplating: an external power source forces electrons to the positive anode, causing metal ions to deposit onto the cathode. This duality is why specifying the cell type is crucial. We at Xuboti often explain this to our clients, many of whom are electrochemical engineers or water treatment equipment owners, primarily from Europe, North America, and Japan.
What are Anodes Used For?
Are you thinking "Okay, I get the basic definition, but where do I see anodes in action?, what’s it used for?
Anodes have diverse applications, including batteries, corrosion protection (sacrificial anodes), electroplating, electrolysis for chemical production, and water treatment.

Exploring the Wide Range of Anode Applications
Let’s break down some key uses:
| Application | Description | Example |
|---|---|---|
| Batteries | Provide electrons in a spontaneous chemical reaction. | Alkaline, lithium-ion batteries |
| Corrosion Protection4 | Sacrificial anodes corrode preferentially, protecting a more valuable metal. | Zinc anodes on ships’ hulls |
| Electroplating | Deposit a thin layer of metal onto another surface. | Chrome plating on car bumpers |
| Electrolysis5 | Drive non-spontaneous chemical reactions using electricity. | Chlorine production, aluminum production |
| Water Treatment6 | Generate oxidants for disinfection and contaminant removal. | Titanium anodes in swimming pool chlorinators |
My company, Xuboti, specializes in manufacturing titanium anodes, particularly for water treatment and electrolysis. These anodes are known for their high catalytic efficiency, durability, and cost-effectiveness. We offer customizable products with a MOQ of 1, and a 7-day fast delivery, which is crucial for clients like Victor, who values delivery time, quality, and price.
What is the Main Function of the Anode?
Wondering what the core job of an anode is, stripped down to its essence? What’s the one thing it’s designed to do?
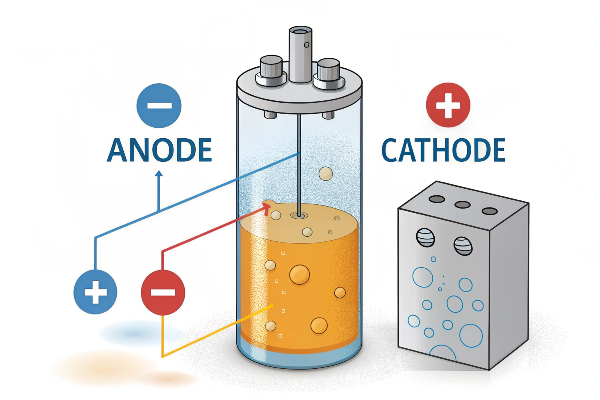
The main function of an anode is to facilitate oxidation, the loss of electrons, within an electrochemical cell7.
The Core Function: Oxidation
Let’s simplify it further:
| Concept | Description |
|---|---|
| Oxidation | The loss of electrons by a substance. |
| Anode’s Role | To be the site where oxidation always occurs. |
Regardless of whether it’s a battery, an electroplating setup, or a water treatment system, the anode always hosts the oxidation reaction. Everything else – the polarity, the specific application – stems from this fundamental function. I often emphasize this to potential clients, many of whom are used to taking the lead in conversations and are very confident. It’s important to establish this fundamental understanding before discussing specific product features.
What is an Example of an Anode in Real Life?
Itching for a tangible, everyday example? Want to see an anode outside of a textbook diagram?
A common real-life example of an anode is the negative terminal of a discharging alkaline battery8. Another example, more relevant to my work, is the titanium anode used in swimming pool chlorinators.

Everyday Anodes and Industrial Applications
Let’s see where we find anodes:
| Context | Anode Example | Function |
|---|---|---|
| Everyday Life | Negative terminal of a discharging AA battery | Zinc inside the battery loses electrons (oxidizes). |
| Industrial | Titanium anode in a swimming pool chlorinator | Oxidizes chloride ions to produce chlorine for disinfection. |
| Marine | Zinc or aluminum "sacrificial anode" on a ship’s hull | Corrodes preferentially, protecting the steel hull. |
The battery example is familiar to everyone. However, at Xuboti, we focus on industrial applications. Our titanium anodes, used in swimming pool chlorinators and other water treatment systems, are a prime example. These anodes oxidize chloride ions in the water, producing chlorine, which acts as a disinfectant. We often deal with clients who, like Victor, lack specific product knowledge and face the challenge of finding reliable suppliers. These real-world examples help bridge that knowledge gap.
Conclusion
Anodes, while seemingly complex, are fundamental components in various electrochemical processes. Understanding their core function – facilitating oxidation – is key to grasping their diverse applications, from batteries to water treatment. I hope this blog helps.
-
Oxidation is a fundamental concept in chemistry. This resource will help clarify its meaning and significance in various reactions. ↩
-
Galvanic cells are fundamental in energy storage and conversion. Discover more about their principles and applications through this resource. ↩
-
Electrolytic cells play a crucial role in various industrial processes. Learn more about their functions and uses by checking this link. ↩
-
Understanding corrosion protection can help you appreciate how sacrificial anodes safeguard valuable metals, enhancing their longevity. ↩
-
Exploring electrolysis will reveal its vital role in various industries, including chemical production and water treatment. ↩
-
Discovering water treatment methods will highlight the importance of anodes in ensuring clean and safe water for various applications. ↩
-
Learn about the components of electrochemical cells to understand how anodes and cathodes work together in various technologies. ↩
-
Explore this link to understand how the negative terminal functions as an anode in batteries, enhancing your knowledge of electrochemistry. ↩





