Electroplating is a fascinating process, one that’s both incredibly complex and wonderfully simple. It’s a technique that has revolutionized countless industries, from jewelry making to automotive manufacturing. But despite its widespread use, there’s one question that often pops up: In electroplating, which is the cathode? Let’s dive into the heart of the matter.
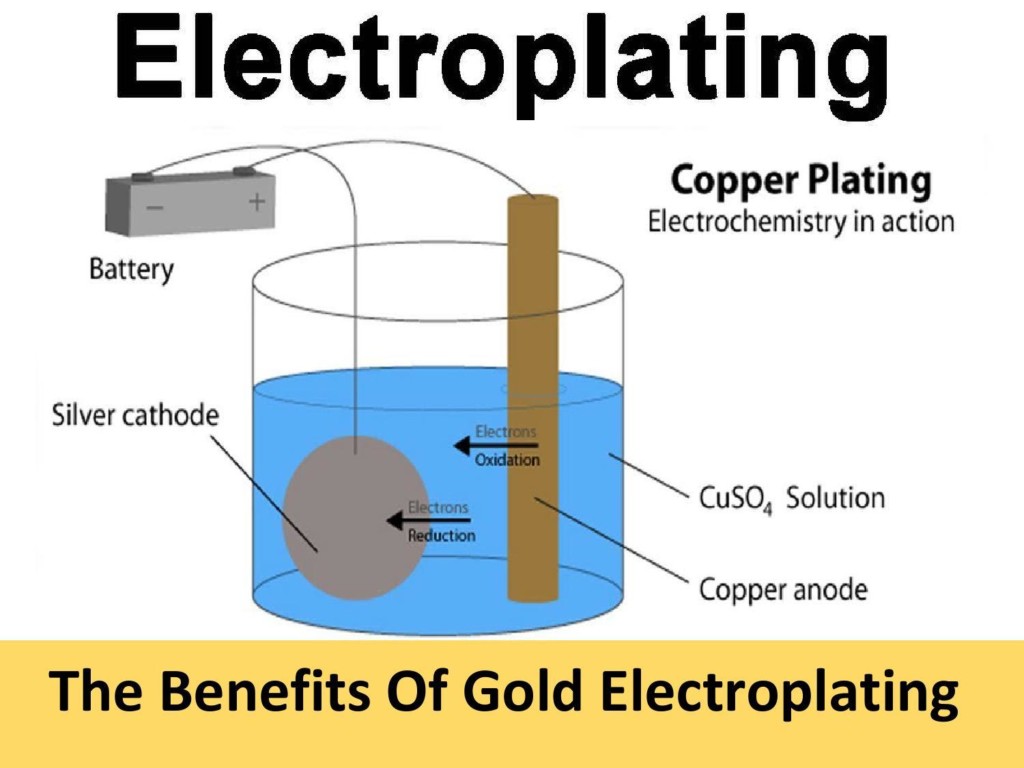
In the world of electroplating, the cathode is the object that is to be plated. This might seem counterintuitive at first glance, especially if you’re not familiar with the principles of electrochemistry. But there’s a very good reason for this setup, and understanding it requires a brief foray into the principles behind electroplating.
The Basic Principle of Electroplating
Electroplating works by using an electric current to reduce dissolved metal cations so that they form a thin coherent metal coating on an electrode. The process involves two electrodes: the cathode, or the part that receives the plating, and the anode, which is made of the metal to be plated onto the cathode. The entire system is immersed in an electrolyte solution that contains one or more metal salts as well as other ions that permit the flow of electricity.
The Role of the Cathode in Electroplating
Why is the object being plated the cathode? It’s all about the flow of electrons and the desired outcome of the electroplating process. When an electric current is passed through the system, it moves from the anode to the cathode. This current flow causes the metal ions in the electrolyte solution to be reduced (gain electrons) and deposit onto the surface of the cathode, thereby plating it with a thin layer of metal.
The Magic Behind the Process
The process of electroplating is akin to alchemy. Through the application of electrical current, metal ions in a solution are coaxed onto the surface of the cathode, transforming it with a layer of precious metal. This transformation is not just superficial; it alters the very essence of the object, imbuing it with new properties. It’s a meticulous dance of chemistry and electricity that requires precision and understanding to execute perfectly.

Each variable in the electroplating process, from the composition of the electrolyte to the intensity of the current, plays a critical role in determining the outcome. By fine-tuning these elements, experts can control the thickness, adherence, and overall quality of the metal coating. This level of control makes electroplating a powerful tool for customization and innovation in materials science.
Choosing the Right Cathode Material
Selecting the appropriate cathode material is pivotal in the electroplating process. The material must not only be conductive but also compatible with the desired metal coating. This compatibility ensures that the electroplated layer adheres smoothly and uniformly, providing the desired aesthetic or functional characteristics. The choice of cathode material is a foundational decision that influences the success of the entire plating endeavor.

Moreover, the cathode’s surface must be meticulously prepared to guarantee the electroplating quality. This preparation may involve processes such as cleaning, etching, or even applying a base layer to promote better adhesion of the metal coating. Such attention to detail ensures that the final product meets the stringent standards of durability, appearance, and performance required by various industries.
The Cathode’s Influence on Electroplating Quality
The cathode’s role in electroplating extends beyond being a mere recipient of the metal layer. Its properties and condition directly impact the uniformity and quality of the electroplated coating. A well-prepared cathode surface ensures that the deposited metal layer is smooth, adherent, and free of defects. This is crucial for applications where precision and reliability are paramount.

Furthermore, the cathode material’s composition and shape can significantly affect the efficiency of the electroplating process. An optimally chosen cathode material enhances the electrical conductivity, facilitating a more uniform metal deposition. This not only improves the quality of the coating but also increases the efficiency of the electroplating process, leading to better outcomes with less waste.
Applications of Electroplating
Electroplating finds its applications in a myriad of industries, each benefiting from the unique properties it imparts to the treated objects. In the automotive sector, electroplating is used to enhance corrosion resistance and wear properties of metal parts, ensuring longevity and reliability under harsh conditions. This technique also adds aesthetic appeal to automotive finishes, contributing to the overall design and value of the vehicle.

In electronics, electroplating is indispensable for creating conductive pathways on circuit boards and components. By depositing a thin layer of precious metals like gold or silver, electroplating ensures superior conductivity and resistance to oxidation. This process is crucial for the manufacturing of high-performance and reliable electronic devices, highlighting electroplating’s role in advancing technology and innovation.
Conclusion: The Cathode’s Central Role
In conclusion, the cathode in electroplating is not just a piece of the puzzle—it’s the canvas upon which the art of electroplating is performed. This process, steeped in science and technology, allows us to manipulate materials at a fundamental level, providing enhancements that go beyond the surface. So, the next time you look at a shiny, metal-plated object, remember the crucial role played by the cathode in creating that beauty and functionality.





