Electroplating is a fascinating process, transforming everyday objects by coating them with layers of precious metals. It’s like magic, but instead of wands and spells, we use anodes and electrolytes. Today, I’m taking you on an insider’s journey into the world of electroplating anodes, the unsung heroes of this alchemical transformation.
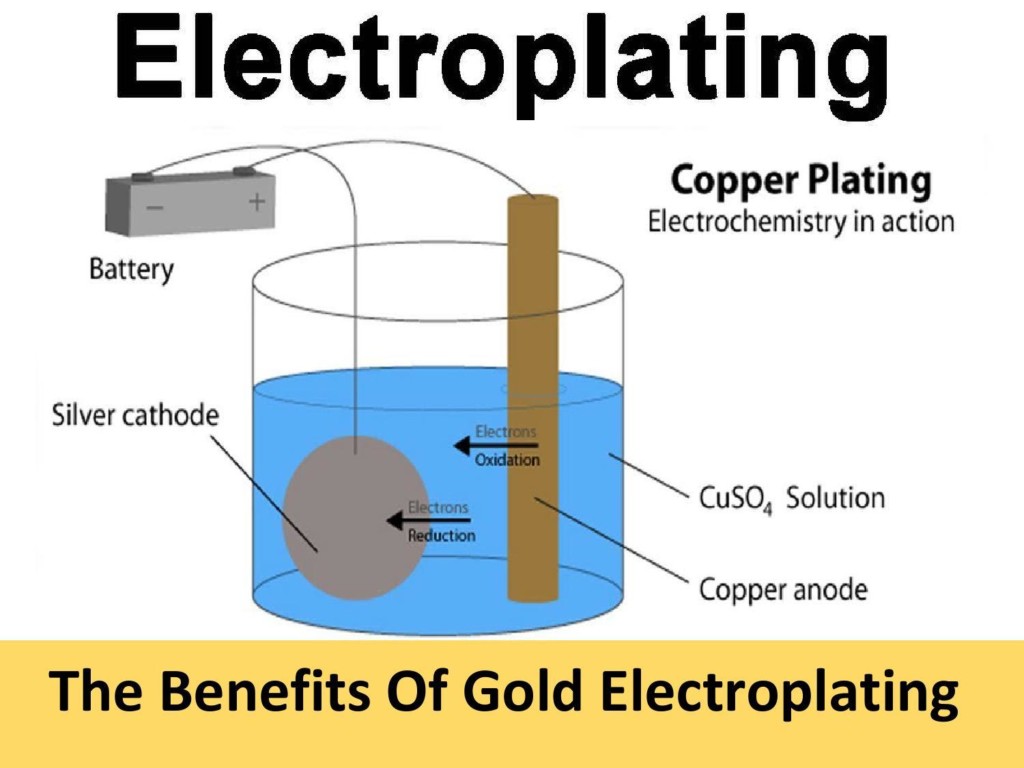
Electroplating anodes, at their core, are conductive materials designed to dissolve into the electrolyte solution, depositing their atoms onto the surface of a cathode (the part being plated). These anodes are not just any materials; they are carefully selected based on their ability to improve the durability, appearance, and functionality of the plated object.
Diving deeper, electroplating anodes are typically made from metals that are intended to be deposited on the substrate. For instance, in our operations at Shaanxi Xubo Titanium Metal Technology Co., Ltd., we specialize in creating titanium anodes coated with various precious metals, including ruthenium, iridium, platinum, and tantalum. Each of these coatings serves a unique purpose and application in the electroplating process.
Why Use Titanium Anodes?
Titanium anodes are the backbone of advanced electroplating technologies. Their popularity isn’t by chance but a result of their superior properties. Firstly, titanium’s exceptional durability and resistance to corrosion make it an ideal substrate for the anode. When coated with precious metal oxides like RuO2, IrO2, Pt, and Ta2O5, titanium anodes become highly efficient at conducting electricity and catalyzing the electroplating process.
The Role of Precious Metal Coatings
Precious metal coatings are not just a layer; they’re the heart of the electroplating process. RuO2 and IrO2, for example, are chosen for their robustness in chlorine and oxygen evolution. This specificity ensures that each project has the ideal anode for its unique requirements, optimizing every electroplating session for maximum efficiency.

Pt coatings stand out for their high catalytic activity. They’re particularly useful in water treatment and ionized water electrolysis. This high performance translates to better durability and efficiency in the electroplating process. As a result, items plated using Pt-coated anodes are more resistant to wear and corrosion, extending their lifespan significantly.
Tantalum coatings, though less commonly discussed, play a crucial role in certain niches. They offer exceptional resistance to acidic environments, making them perfect for specialized applications. The choice of metal coating directly impacts the anode’s effectiveness, tailoring each anode to meet specific industrial demands with unparalleled precision.

The Science Behind the Coatings
At the core of our coated titanium anodes is a commitment to reducing cell voltage. This not only saves energy but also increases the anode’s lifespan. Our advanced coating technology ensures that the electroplating process is not just efficient but also sustainable, keeping operational costs low without compromising on quality.
Our coatings are engineered to maintain the purity of the electrolysis system. This is vital for preventing contamination and ensuring the high quality of the final plated product. It’s a delicate balance, ensuring that our anodes contribute to a clean, efficient process. This focus on purity is what sets our anodes apart, offering a clear advantage in any electroplating setup.

Moreover, the ability to reuse and recoat the titanium substrate is a testament to the sustainability of our approach. This not only reduces waste but also offers a cost-effective solution for long-term operations. Our anodes are designed for longevity, embodying our commitment to innovation and environmental stewardship in the electroplating industry.
Applications Beyond Imagination
Our coated titanium anodes find their way into a myriad of applications, each benefiting from the unique properties of the coatings. Whether it’s in the recovery of copper from etching liquids or the disinfection of swimming pools, these anodes are at the forefront of efficiency and effectiveness, pushing the boundaries of what’s possible in electroplating.

In the chlor-alkali industry, our anodes play a crucial role. They’re not just components; they’re catalysts for change, enabling cleaner, more efficient production processes. This versatility is a hallmark of our product range, highlighting the adaptability and breadth of applications for our coated anodes.
Even more fascinating is the use of our anodes in water heaters and hydrometallurgy. These fields require precise control and high efficiency, qualities that our anodes deliver consistently. It’s a testament to the ingenuity behind our products, showcasing the limitless potential of our coated titanium anodes in transforming industries far and wide.

A Commitment to Innovation
At Shaanxi Xubo, innovation is at the heart of what we do. We continuously explore new materials and coatings to enhance the electroplating process. Our R&D efforts are focused on developing electrodes that offer higher chlorine evolution efficiency, better resistance in acidic environments, and materials suited for mixed acid systems. This commitment to innovation ensures that our clients receive anodes that are not only fit for their current needs but also adaptable to future challenges.

Conclusion
Electroplating anodes are more than just pieces of metal; they are the result of sophisticated engineering and material science. Through the use of titanium substrates and precious metal coatings, we’ve unlocked new possibilities in electroplating, offering solutions that are energy-efficient, durable, and environmentally friendly. As we continue to push the boundaries of what’s possible, one thing remains clear: the future of electroplating shines bright, and it’s coated in precious metals.
So, the next time you admire a piece of electroplated jewelry or hardware, remember the complex interplay of materials and technology that made it possible. Behind every lustrous finish lies the science of electroplating anodes, the true stars of the show.





