Have you ever wondered how some metal objects get that shiny, perfect coating? It might seem like magic, but it’s science, specifically a process called electroplating.
Electroplating for class 8 is a scientific process that uses electricity to put a thin layer of one metal onto the surface of another object. This makes the object look better or protects it from rust.
Understanding this process isn’t as complicated as it sounds. Let’s break down exactly how it works and why it’s so important in our daily lives. I remember first learning about this in school, and it felt like unlocking a secret about how everyday things are made.
What is electroplating in simple words?
Confused about how a dull metal spoon can become sparkling silver? You might think it’s painted, but often, it’s a clever process using electricity and chemistry.
Simply put, electroplating1 is like giving an object a very thin metal skin. We use an electric current to deposit dissolved metal ions from a solution onto the surface of the object.

Let’s dive a bit deeper into how this actually happens. It involves a few key components working together. Think of it like a carefully controlled science experiment happening right before your eyes, but often on an industrial scale.
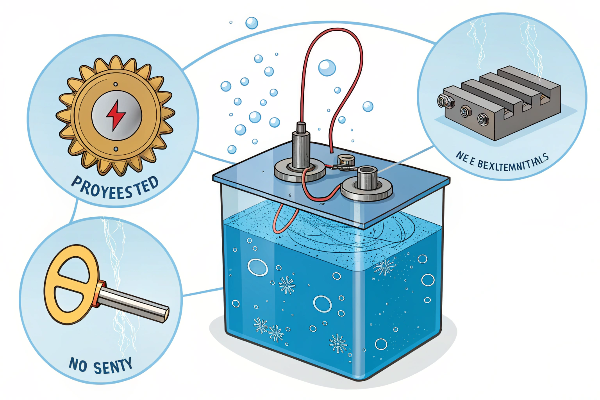
The Electroplating Setup
To understand electroplating, we need to know the basic parts involved. It usually takes place in a bath or tank containing a special liquid.
- Anode: This is the metal that we want to use as the coating (like chromium or nickel). It’s connected to the positive terminal (+) of a power source.
- Cathode: This is the object we want to coat (like an iron spoon). It’s connected to the negative terminal (-) of the power source.
- Electrolyte: This is a special solution, often containing dissolved salts of the coating metal (like copper sulfate if we’re copper plating).
The Process Explained
When we turn on the power supply, an electric current flows through the electrolyte2. This current causes a chemical reaction. Metal atoms from the anode3 lose electrons and become positively charged ions, dissolving into the electrolyte. At the same time, these positive metal ions in the electrolyte are attracted to the negatively charged cathode (the object). When they reach the object, they gain electrons and turn back into solid metal atoms, forming a thin, even layer on the object’s surface. It’s a precise transfer of metal, atom by atom.
| Component | Role in Electroplating | Connected To | Example |
|---|---|---|---|
| Anode | Source of the coating metal | Positive (+) Terminal | A piece of Copper |
| Cathode | Object to be coated | Negative (-) Terminal | An Iron key |
| Electrolyte | Conducts electricity, contains ions | The Bath | Copper Sulfate Solution |
| Power Supply | Provides the electric current | Anode & Cathode | Battery/DC Source |
Why is electroplating done class 8?
Ever seen a rusty old bike and a shiny new one? Often, the difference isn’t just age; it’s a protective layer applied through processes like electroplating. Without it, many metal objects would quickly degrade.
Electroplating is done mainly for two reasons: to protect the underlying object from corrosion4 (like rust) and to make the object look more attractive5 with a shiny or decorative finish.
There’s more to it than just looks and rust prevention, though. Let’s explore the various benefits that make electroplating such a widely used technique in manufacturing and industry6.
Key Benefits of Electroplating
Electroplating offers several advantages, making it valuable for many applications.
- Corrosion Resistance7: This is a big one. Many metals, like iron and steel, rust easily when exposed to air and moisture. A layer of a non-corrosive metal like chromium, nickel, or zinc acts as a barrier, preventing rust and extending the object’s lifespan. Think about bathroom taps – they need to resist water constantly.
- Appearance Enhancement8: A thin layer of gold, silver, or chromium can make a less expensive base metal look much more appealing and valuable. This is common in jewelry, cutlery, and decorative items. I remember getting a "silver" bracelet as a kid; it was likely silver-plated copper!
- Improved Hardness and Durability: Some coatings, like chromium, are very hard and resistant to wear and tear. Electroplating can make softer materials more durable for tools or machine parts.
- Increased Electrical Conductivity9: Sometimes, a layer of a highly conductive metal like silver or copper is electroplated onto components used in electronics to improve their performance.
Trade-offs to Consider
While beneficial, electroplating isn’t perfect.
- Environmental Concerns: The process often involves strong chemicals and produces waste that needs careful handling and disposal to avoid pollution.
- Cost: While it can make things look expensive, the process itself requires equipment, chemicals, and energy, adding to the manufacturing cost.
- Adhesion Issues: If not done correctly, the plated layer might peel or flake off over time.
| Benefit | Description | Example |
|---|---|---|
| Corrosion Resistance | Protects base metal from rust or tarnishing. | Zinc plating on nuts/bolts |
| Appearance Enhancement | Improves look with shiny/colored metal layer. | Gold plating on jewelry |
| Increased Hardness | Makes the surface tougher and wear-resistant. | Chromium plating on tools |
| Electrical Conductivity | Improves the flow of electricity. | Silver plating on contacts |
| Cost Reduction (Material) | Using cheaper base metal with expensive coating. | Silver-plated cutlery |
What are the two metals that can be electroplated Class 8?
Thinking about electroplating, you might wonder which metals are involved. Can you plate any metal onto another? Not quite, but many common ones are used.
While many metals can be involved, two very common examples taught in Class 8 are copper10 (often used in experiments) and chromium11 (known for its shiny finish on taps and car parts).

Copper and chromium are just the beginning, though. Many different metals are used for electroplating, each chosen for its specific properties. Let’s look at some others and why they are selected.
Common Metals Used for Plating
Different metals offer different benefits when used as a coating. Here are a few key examples:
- Chromium: Extremely popular for its bright, shiny appearance (like on car bumpers or bathroom fixtures) and excellent hardness and corrosion resistance. It provides a durable, protective, and decorative finish.
- Nickel: Often used as an underlayer before chromium plating or as a finish itself. It provides good corrosion resistance, wear resistance, and a bright appearance. Sometimes used in coins.
- Zinc: Primarily used for corrosion protection, especially on steel (this process is called galvanization, often done via electroplating). Think of screws, nuts, bolts, and sheet metal used outdoors. It sacrifices itself to protect the steel underneath.
- Copper: Used both decoratively and functionally. It’s an excellent conductor of electricity, so it’s used in electronics. It also serves as a good base layer for other plating metals like nickel12 or silver. I remember doing a simple copper plating experiment in science class using a coin and a copper sulfate solution.
- Tin: Often used to coat steel cans for food packaging (tin cans) because it’s non-toxic and resists corrosion from food acids. Also used in electronics for soldering.
- Gold and Silver: Used mainly for decorative purposes in jewelry and high-end tableware due to their appearance and value. Also used in electronics for their excellent conductivity and resistance to corrosion on contacts.
Choosing the Right Metal
The choice of metal depends entirely on the desired outcome.
| Plating Metal | Key Property | Common Use | Reason |
|---|---|---|---|
| Chromium | Hardness, Shine | Car parts, Taps | Durability13, Appearance, Corrosion Res. |
| Nickel | Corrosion Res.14, Base | Underlayer, Coins | Protection, Preparation for Chrome |
| Zinc | Corrosion Res. | Nuts, Bolts, Outdoor Steel | Sacrificial protection (Galvanizing) |
| Copper | Conductivity15, Base | Electronics, Pre-plating layer | Electrical properties, Adhesion base |
| Tin | Non-toxic, Corr. Res | Food Cans, Soldering | Food safety, Solderability |
| Gold/Silver | Appearance, Value | Jewelry, Electronics Contacts | Decoration, Conductivity, Inertness |
How is electroplating used in everyday life?
Think electroplating is just some obscure industrial process? Look around you! From the spoon you eat with to the car you ride in, electroplated objects are everywhere, often hiding in plain sight.
Electroplating is used constantly in everyday life. Examples include shiny bathroom taps (chromium plating), jewelry (gold/silver plating), tin cans for food (tin plating16), and car parts like bumpers (chromium plating).

The applications are incredibly diverse, impacting almost every aspect of modern living. Let’s explore some specific areas where electroplating plays a crucial role, making things work better, last longer, or simply look nicer.
Where We See Electroplating
You interact with electroplated items more often than you probably realize.
- Household Fixtures: Bathroom taps, showerheads, kitchen faucets, and cabinet handles are often chrome-plated17 over brass or zinc. This provides a shiny look and protects against water damage and wear.
- Automotive Industry: Car bumpers, grilles, wheel rims, and trim pieces frequently use chromium plating for aesthetics and durability against weather and road debris. Zinc plating protects many nuts, bolts, and underbody components from rust.
- Jewelry and Accessories: Less expensive metals like copper or nickel are often plated with gold or silver to create affordable yet attractive jewelry, watches, and belt buckles. [Personal story about realizing a favorite ‘gold’ necklace was plated].
- Electronics: Connectors, pins, and circuit board components are often plated with gold, silver, or tin to improve electrical conductivity and prevent corrosion, ensuring reliable performance.
- Cutlery and Tableware: Forks, spoons, and knives are sometimes silver-plated or chrome-plated over stainless steel or other base metals for appearance and corrosion resistance.
- Food Packaging: Steel cans are coated with a thin layer of tin to prevent the steel from rusting and reacting with the food contents.
Beyond the Obvious
There are less visible but equally important uses too.
| Application Area | Specific Example | Plating Metal(s) Used | Purpose |
|---|---|---|---|
| Household | Bathroom Faucet | Chromium, Nickel | Appearance18, Corrosion Resistance19 |
| Automotive | Car Bumper | Chromium | Appearance, Durability20, Corrosion Res. |
| Jewelry | Gold-plated Ring | Gold | Appearance, Cost Reduction |
| Electronics | Circuit Board Connector | Gold, Tin, Silver | Conductivity, Corrosion Resistance |
| Tableware | Silver-plated Spoon | Silver | Appearance, Corrosion Resistance |
| Food Packaging | Tin Can | Tin | Corrosion Resistance, Food Safety |
| Industrial Tools | Chrome-plated Wrench | Chromium | Hardness, Durability |
| Aerospace | Engine Components | Nickel, Cadmium | Wear Resistance, Corrosion Resistance |
Can I electroplate plastic?
We’ve talked a lot about plating metal onto metal. But what about plastic? It doesn’t conduct electricity, so surely you can’t electroplate it, right?
Yes, you can electroplate plastic21! However, since plastic doesn’t conduct electricity, it requires special pre-treatment steps first to make its surface conductive before the standard electroplating process can begin.

This opens up a whole world of possibilities, allowing us to combine the lightweight nature of plastic with the appearance and surface properties of metal. Let’s look at how this seemingly impossible task is achieved.
Making Plastic Conductive22
The key challenge is that plastic is an insulator. Electricity won’t flow through it to allow metal ions to deposit. So, we need to make the surface conductive first. This usually involves a multi-step process:
- Etching: The plastic surface is chemically treated (often with strong acids like chromic acid) to create microscopic pits and pores. This roughens the surface, making it easier for subsequent layers to stick.
- Neutralization & Conditioning: Acid residues are removed, and the surface is conditioned.
- Activation/Seeding: The surface is treated with a catalyst, often involving palladium chloride. Tiny particles of palladium get embedded in the pores created during etching.
- Electroless Plating23: Before electroplating, a thin conductive metal layer (usually nickel or copper) is deposited chemically, without using an external electric current. This is called ‘electroless plating’. The palladium particles act as sites for this chemical reaction to start. This initial thin metal layer now makes the entire plastic surface conductive.
The Final Electroplating Step
Once the plastic object has this thin, conductive metal layer from electroless plating, it can be treated just like a metal object in a standard electroplating bath. It becomes the cathode, and layers of copper, nickel, chromium, or other metals can be built up on top of the initial electroless layer.
| Step | Purpose | Method | Result |
|---|---|---|---|
| 1. Etching | Roughen surface for adhesion | Chemical treatment (e.g., Chromic Acid) | Microscopic pits and pores |
| 2. Activation | Prepare surface for metal deposition | Apply catalyst (e.g., Palladium Chloride) | Catalyst particles embedded in surface |
| 3. Electroless Plating24 | Create initial conductive layer | Chemical deposition (e.g., Nickel/Copper) | Thin, conductive metal coating |
| 4. Electroplating25 | Build up final metal layers | Standard electroplating process | Desired metal finish (e.g., Chrome) |
This process, often called "Plating on Plastics26" (POP), is widely used for things like shiny car grilles, emblems, electronic device casings, and decorative parts where lightweight plastic is desired but a metallic look is needed. It combines the best of both worlds!
Conclusion
So, electroplating is a fascinating process using electricity to coat objects with metal. It protects items, makes them look better, and is used in countless everyday objects around us.
-
Explore this link to gain a deeper understanding of electroplating, its applications, and its significance in various industries. ↩
-
Learn about the role of electrolytes in electroplating processes and how they contribute to the quality of the coating. ↩
-
Discover the importance of the anode in electroplating and how it influences the coating process. ↩
-
Understanding corrosion protection can enhance your knowledge of electroplating’s significance in various industries. ↩
-
Discover the aesthetic benefits of electroplating and how it enhances product appeal in manufacturing. ↩
-
Explore the diverse applications of electroplating in industry to see its impact on modern manufacturing processes. ↩
-
Understanding corrosion resistance can help you appreciate how electroplating protects metals from rust and extends their lifespan. ↩
-
Explore how electroplating enhances the aesthetic appeal of various items, making them look more valuable and attractive. ↩
-
Learn about the importance of electrical conductivity in electronics and how electroplating improves performance. ↩
-
Explore the unique properties of copper in electroplating and its applications in various industries. ↩
-
Learn about chromium’s advantages in electroplating, including its durability and aesthetic appeal. ↩
-
Discover how nickel enhances electroplating, providing corrosion resistance and a bright finish. ↩
-
Learn about the importance of metal durability to ensure the right choice for your specific needs and projects. ↩
-
Understanding corrosion resistance can help you choose the right metal for durability and longevity in various applications. ↩
-
Exploring conductivity in metals is crucial for applications in electronics and electrical engineering. ↩
-
Explore the critical role of tin plating in food safety and packaging, ensuring your food stays fresh and uncontaminated. ↩
-
Discover how chrome-plated fixtures enhance durability and aesthetics in your home, making them a smart choice for any bathroom or kitchen. ↩
-
Explore how plating enhances aesthetics and value in everyday items, making them more appealing and functional. ↩
-
Understanding corrosion resistance can help you choose the right materials for durability and longevity in various applications. ↩
-
Learn why durability is crucial in automotive parts to ensure safety and performance over time, enhancing vehicle reliability. ↩
-
Discover the innovative techniques used to electroplate plastic, transforming its surface properties and expanding its applications. ↩
-
Learn about the essential steps to make plastic conductive, enabling the electroplating process and enhancing material versatility. ↩
-
Explore the process of electroless plating, a crucial step in electroplating plastic that ensures a successful metal deposition. ↩
-
Understanding Electroless Plating can enhance your knowledge of metal coating processes and their applications. ↩
-
Learning about Electroplating will provide insights into its applications in manufacturing and design, crucial for various industries. ↩
-
Exploring the benefits of Plating on Plastics can reveal innovative uses in various industries, enhancing product design. ↩





