Feeling frustrated by relentless rust and corrosion eating away at your valuable metal structures? Wondering if there’s a way to stop this destructive process before it’s too late?
Cathodic protection (CP) is a technique used to control the corrosion of a metal surface by making it the cathode of an electrochemical cell. This is achieved by providing an alternative pathway for the corrosive current to flow, thus protecting the target metal.
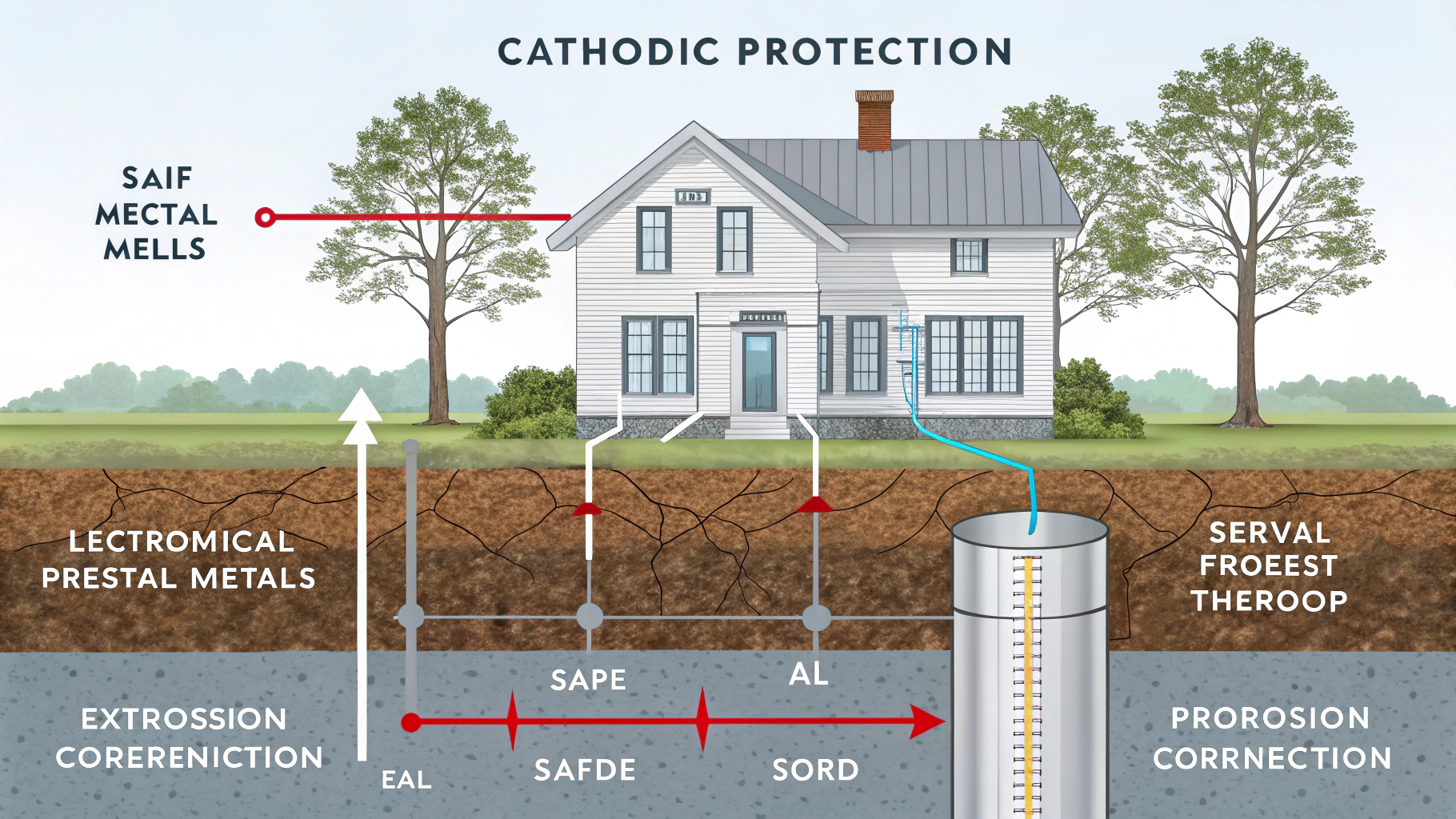
It seems like a complex solution, but stick with me. We’ll break down the core concepts and see exactly how this powerful technique keeps metal structures safe from the ravages of corrosion. Let’s start the explaination!
What are Two Types of Cathodic Protection?
Tired of seeing your metal investments crumble under the relentless attack of corrosion? Want a solution that’s both effective and long-lasting?
The two main types of cathodic protection1 are sacrificial (or galvanic)2 and impressed current3. Sacrificial CP uses a more reactive metal anode, while impressed current CP uses an external power source and a less reactive anode.4

Let’s delve deeper into how these methods work and when to best apply them.
Diving Deeper into the Types of CP
Both types achieve the same outcome, that is stopping corrosion, but they operate differently. Here’s a more detailed breakdown:
-
Sacrificial (Galvanic) Cathodic Protection:
- This method uses a more "active" metal (like zinc, aluminum, or magnesium) as an anode. 4
- This active metal corrodes preferentially, sacrificing itself to protect the target metal structure.
- It’s like a bodyguard taking a bullet for the VIP.
- No external power source is needed, making it simpler to install and maintain.
- It is Limited in its current output, and the anodes need periodic replacement.
-
Impressed Current Cathodic Protection (ICCP):
- This method uses an external DC power source (a rectifier) to drive the protective current.4
- The anode is usually made of a less reactive material, such as titanium, graphite, or mixed metal oxide. 5
- The rectifier converts AC power to DC power.
- It’s like having a force field powered by an external generator.
- ICCP systems can provide higher current outputs, making them suitable for large structures.
- It needs more complex installation and ongoing monitoring.
| Feature | Sacrificial CP | Impressed Current CP |
|---|---|---|
| Power Source | None (self-powered) | External DC power source (rectifier) |
| Anode Material | Reactive metals (Zn, Al, Mg) | Less reactive materials (graphite, titanium, MMO) |
| Current Output | Lower | Higher |
| Structure Size | Smaller structures, pipelines | Larger structures, pipelines, tanks |
| Maintenance | Anode replacement | Monitoring, rectifier adjustment |
| Installation | Simpler | More complex |
| Cost | lower, need replacement. | Higher initial cost, Lower long-term cost |
What is Cathodic Coating?
Is "cathodic coating" a thing in your field? Have you been confused with similar terminologies?
Cathodic coating is NOT one type of cathodic protection. It describes a metallic coating that is cathodic (more noble) about the base metal.6

It’s essential to clarify that the term "cathodic coating" can be misleading, It is different from cathodic protection.
Diving Deeper into Cathodic Coating
The key to understanding "cathodic coating" lies in the electrochemical relationship between the coating and the underlying metal. The opposite of "cathodic coating" would be "anodic coating", and here is the comparison of them:
-
Cathodic Coating
- A coating is considered cathodic when it is more noble (less reactive) than the substrate material.6
- Common examples include nickel, chromium, and silver coatings on steel.
- If the coating is scratched or damaged, exposing the substrate, the substrate will corrode preferentially. The coating acts as the cathode and accelerates corrosion of the base metal at the point of damage.
- A complete and intact coating is needed.
-
Anodic Coating
- A coating is considered anodic when it is less noble (more reactive) than the substrate material.
- Common examples include zinc (galvanizing) or cadmium coatings on steel.
- If the coating is scratched, the coating itself will corrode preferentially, protecting the substrate. This is similar to the sacrificial anode method of cathodic protection.
- Even if damaged, it can still offer some protection.
| Feature | Cathodic Coating | Anodic Coating |
|---|---|---|
| Nobility | More noble (less reactive) than substrate | Less noble (more reactive) than substrate |
| Example | Nickel, chromium, silver on steel | Zinc (galvanizing), cadmium on steel |
| Corrosion Behavior | Substrate corrodes if coating is damaged | Coating corrodes preferentially if damaged |
| Protection Mechanism | Barrier protection only, no sacrificial action | Barrier protection AND sacrificial action |
It is totally different from cathodic protection. Don’t be confused!
How Does Cathodic Protection Prevent Rusting?
Are you fighting a losing battle against rust? Want to know the secret weapon that can turn the tide?
Cathodic protection prevents rusting by shifting the electrochemical potential of the metal surface to a range where corrosion reactions are thermodynamically unfavorable. It suppresses the anodic dissolution of the metal, which is the primary process in rust formation.7
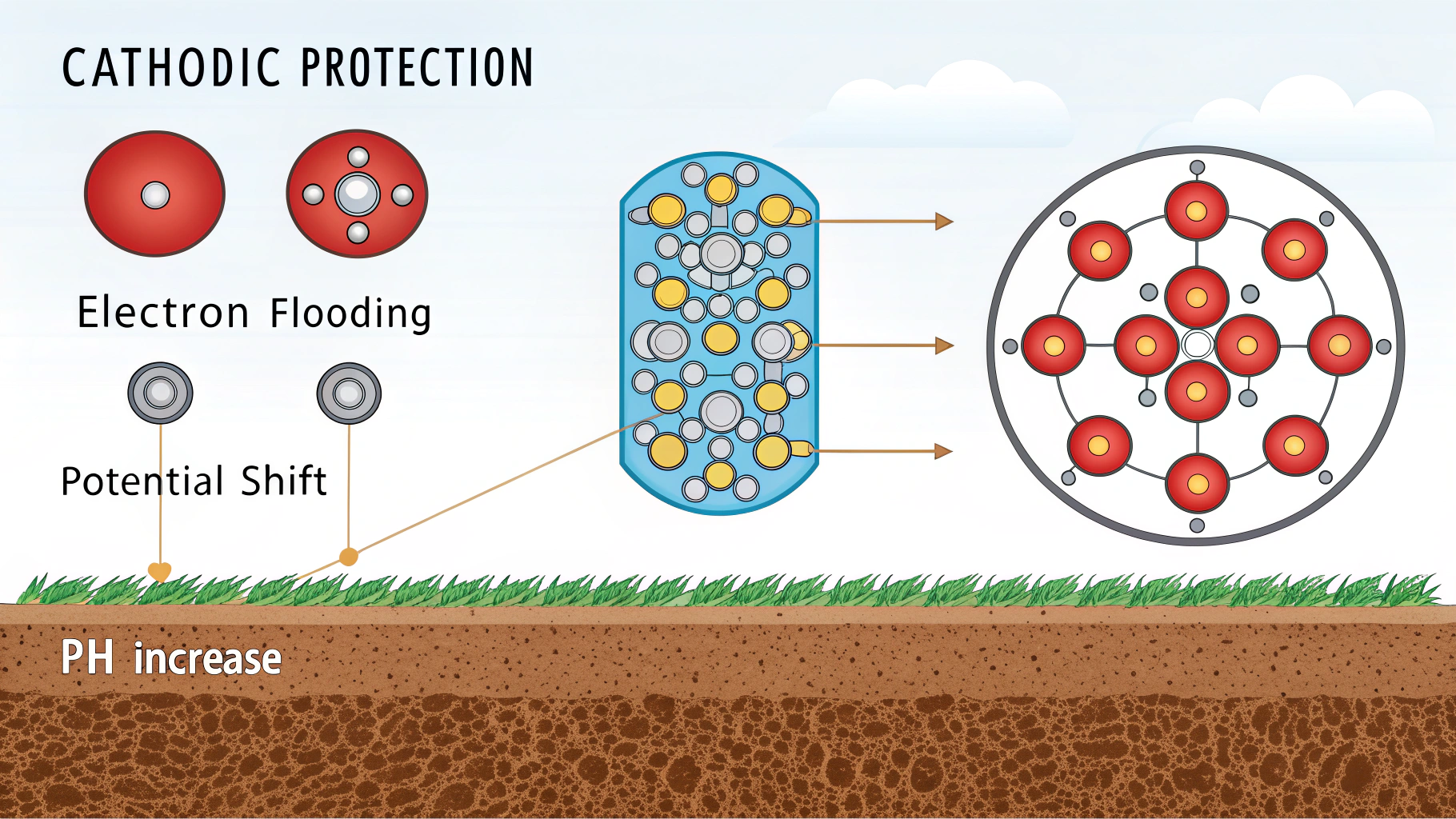
Let’s dig deeper into the underlying electrochemical principles and see how this shift in potential works its magic.
Diving Deeper into the Electrochemical Prevention of Rusting
Rusting is an electrochemical process8. Cathodic protection works by interfering with this process.
-
The Chemistry of Rust:
- Rusting (specifically, the corrosion of iron) involves the oxidation of iron (Fe) to form iron ions (Fe2+ or Fe3+). This is the anodic reaction.
- Simultaneously, oxygen (O2) and water (H2O) are reduced to form hydroxide ions (OH-). This is the cathodic reaction.
- These two reactions are coupled; one cannot occur without the other. The iron ions and hydroxide ions then react further to form various iron oxides and hydroxides, which we see as rust.
-
How CP Changes the Game:
- CP supplies electrons to the metal surface, making it more negatively charged.
- This excess of electrons suppresses the anodic reaction (the dissolution of iron).
- The increased electron concentration also favors the cathodic reaction (reduction of oxygen and water), but this reaction doesn’t harm the metal structure.
- Essentially, CP shifts the metal’s potential to a region where the oxidation of iron is significantly reduced or stopped altogether.
-
Pourbaix Diagrams:
- The effectiveness of CP is often visualized using Pourbaix diagrams9 (potential-pH diagrams).
- These diagrams show the thermodynamically stable phases of a metal in an aqueous environment as a function of potential and pH.
- CP aims to shift the metal’s potential into the "immunity" region of the Pourbaix diagram, where corrosion is not thermodynamically favored.
How Does the Cathodic Protection Work?
Feeling overwhelmed by the science of cathodic protection? Wondering if you can really understand the basics without a PhD in electrochemistry?
Cathodic protection works by introducing a new electrode (the anode) into the corrosion circuit, forcing the structure to be protected to become entirely cathodic, thus suppressing corrosion reactions on its surface.1
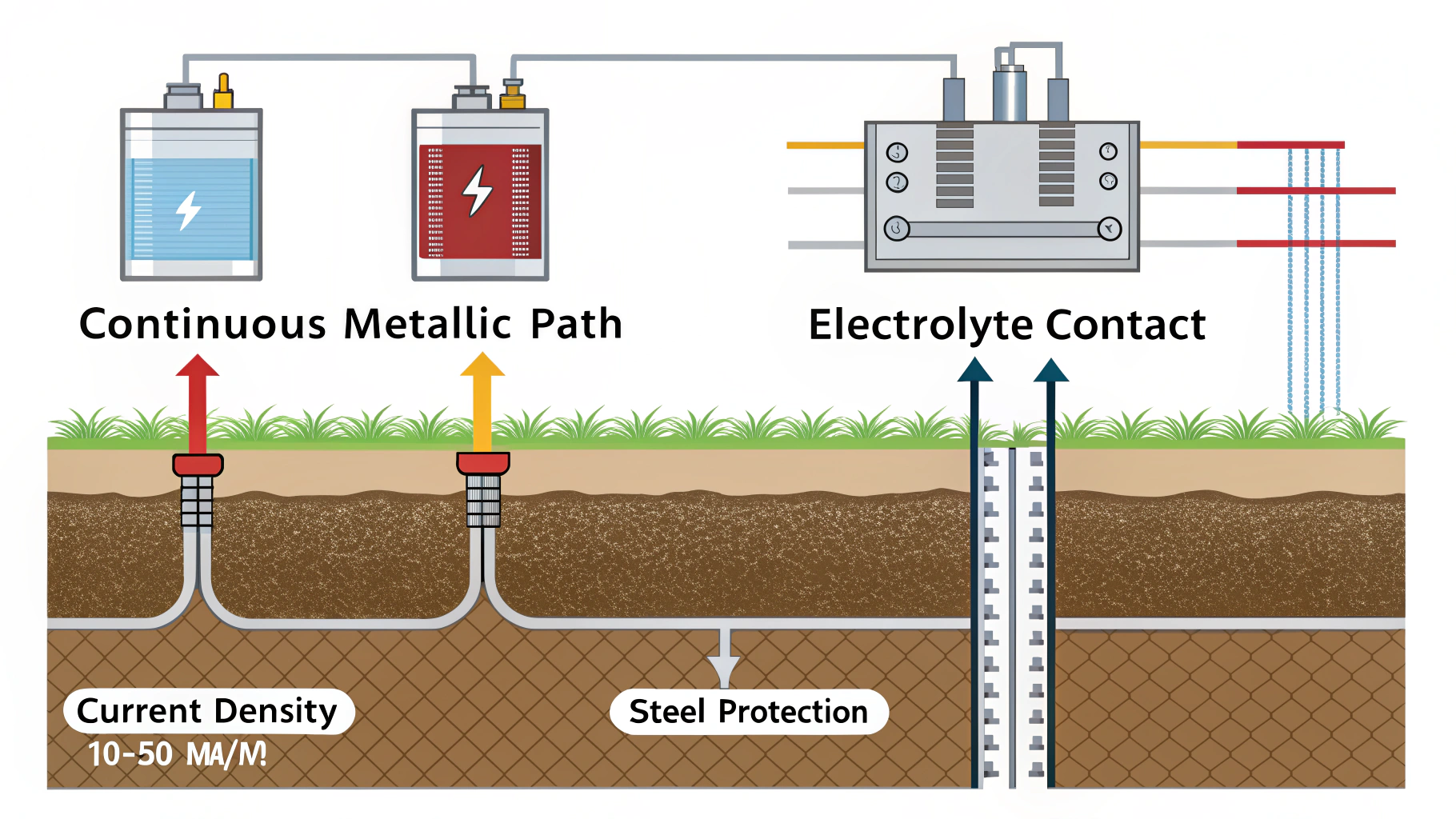
But how does it all fit together in practice? Let’s break down the process into understandable pieces.
Diving Deeper into the CP process.
Here’s a step-by-step look at how it works:
-
The Corrosion Cell:
- Before CP is applied, a metal structure in an electrolyte (like soil or water) naturally forms corrosion cells.
- Some areas become anodic (where corrosion occurs) and some become cathodic (where reduction occurs).
- Electrons flow from the anodic areas to the cathodic areas through the metal, and ions flow through the electrolyte, completing the circuit.
-
Introducing the Anode:
- In CP, a new anode is introduced into the electrolyte. This anode can be either a sacrificial metal or an inert anode connected to a DC power source.
-
Shifting the Current:
- Sacrificial Anode: The sacrificial anode, being more reactive, becomes the primary anode in the system. It corrodes preferentially, releasing electrons that flow to the protected structure.
- Impressed Current: The DC power source forces current to flow from the inert anode through the electrolyte to the structure.
-
Making the Structure Cathodic:
- The influx of electrons from the anode (either sacrificial or impressed current) makes the entire structure cathodic.
- This means the entire surface of the protected structure becomes the site of the reduction reaction (usually oxygen reduction).
- The original anodic areas on the structure are suppressed because they are now receiving electrons rather than losing them.
-
Preventing Corrosion:
- By forcing the entire structure to become cathodic, the anodic dissolution of the metal (corrosion) is halted or significantly reduced.
What are the Requirements for Cathodic Protection?
Thinking about implementing cathodic protection? Wondering if your specific situation is suitable for this technology?
The primary requirements for cathodic protection include a continuous electrolyte, a suitable anode system, a DC power source (for ICCP), and a proper electrical connection between the anode, structure, and power source (if applicable).10
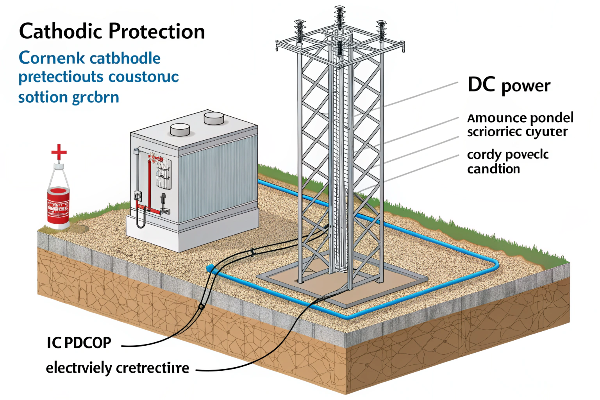
It’s crucial to consider several factors to ensure CP is effective and efficient. Let’s explore these essential prerequisites.
Diving Deeper into CP Requirements
Meeting these requirements is essential for the success of a CP system:
-
Continuous Electrolyte:
- A continuous electrolyte (a substance that conducts electricity due to the presence of ions) is essential for CP to work.10
- This electrolyte, which could be soil, water, or even concrete, provides the pathway for ionic current flow between the anode and the structure being protected.
- If the electrolyte is not continuous, the CP current won’t be able to reach all parts of the structure, leaving some areas unprotected.
-
Anode System:
- The choice of anode system (sacrificial or impressed current) depends on factors like the size of the structure, the environment, and the desired lifespan of the protection.
- Sacrificial anodes must be more electrochemically active than the structure being protected.4
- Impressed current anodes must be durable and able to discharge current without rapid degradation.
-
DC Power Source (for ICCP):
- Impressed current systems require a reliable DC power source, typically a rectifier that converts AC power to DC power.4
- The power source must be able to provide sufficient current to protect the entire structure.
- Voltage and current requirements need to be carefully calculated based on the surface area of the structure and the resistivity of the electrolyte.
-
Electrical Connections:
- All connections between the anode, the structure, and the power source (if applicable) must be electrically conductive and mechanically sound.10
- Poor connections can introduce resistance into the circuit, reducing the effectiveness of the CP system.
- Connections should be regularly inspected and maintained.
-
Structure Continuity:
- The structure needs to be electrically continuous, the welding parts need to be connected with cables.
-
Monitoring and Maintenance:
- Regular monitoring of the CP system is crucial to ensure it’s functioning correctly.
- This typically involves measuring the structure-to-electrolyte potential using reference electrodes.
- Maintenance may include replacing sacrificial anodes, adjusting the output of the rectifier, and repairing any damaged connections.
Conclusion
Cathodic protection is a powerful and versatile technique for preventing corrosion in a wide range of applications. By understanding the basic principles, the different types of systems, and the key requirements, you can apply them.
-
Discover the different types of cathodic protection and find the best solution for your corrosion issues. ↩ ↩
-
Explore this link to understand how sacrificial cathodic protection works and its benefits for metal structures. ↩
-
Learn about impressed current cathodic protection and how it can effectively protect larger structures from corrosion. ↩
-
Cathodic Protection: Types, Working Principles, Advantages ↩ ↩ ↩ ↩ ↩
-
What Are The Different Types of Cathodic Protection Anodes? ↩
-
Discover the electrochemical processes involved in rusting and how to effectively prevent it through various methods. ↩
-
Learn about Pourbaix diagrams to see how they visualize corrosion processes and the effectiveness of cathodic protection. ↩
-
What are the basic requirements of a cathodic protection system? – Quora ↩ ↩ ↩





